Abstract
Occurrence of dynamic left ventricular outflow tract (LVOT) obstruction is not infrequent in critically ill patients, and it is associated with potential danger. Here, we report a case of transient heart failure with hemodynamic deterioration paradoxically induced by extreme dehydration. This article describes clinical features of the patient and echocardiographic findings of dynamic LVOT obstruction and significant mitral regurgitation caused by systolic anterior motion of the mitral valve in a volume-depleted heart.
Dynamic left ventricular outflow tract (LVOT) obstruction is typically observed in patients with hypertrophic cardiomyopathy as a consequence of systolic anterior motion (SAM) of the mitral valve (MV) leaflet and hypertrophied septum. In addition, dynamic intraventricular obstruction with/without mitral regurgitation (MR) has been reported in patients with specific conditions, including acute coronary syndrome,1 after valve surgery,2 stress-induced cardiomyopathy,3 and even in a setting of structurally normal heart with precipitating factors.4,5 Here, we report a case of extreme dehydration in a patient suffering from transient heart failure and hemodynamic deterioration due to dynamic LVOT obstruction and significant MR induced by SAM of the MV, which occurred in a volume-depleted heart.
A 59 year-old Asian female presented with dyspnea (New York Heart Association class III/IV) which had developed and became aggravated over past several days. The patient had medical histories of old cerebrovascular accident and epilepsy. She hemodyhad not taken any food or water for a week due to long-standing anorexia and vomiting associated with her brain disease. Vital signs were notable with regard to hypotension (80/50 mm Hg) and tachycardia (heart rate: 110/min). On physical examination, she appeared tachypneic (respiratory rate: 20/min) with decreased skin turgor and a dry tongue. On auscultation, a systolic murmur (grade IV/VI) was audible at the left lower sternal border. Of note, chest radiography revealed newly developed pulmonary congestion (Fig. 1), which was unexpected. A 12-lead electrocardiography examination showed sinus tachycardia and nonspecific changes of ST-segment and T-wave. Laboratory findings revealed high plasma osmolality (388 mOsm/kg), hypernatremia (plasma sodium concentration: 167 mEq/L), uremia (blood urea nitrogen was 79.5 mg/dL) and increased plasma creatinine level (2.4 mg/dL), which were all suggestive of systemic dehydration. Hemoglobin was decreased to 8.4 g/dL. Serum protein was 7.6 g/dL and albumin was 3.2 g/dL.
The patient underwent transthoracic echocardiography for further evaluation of systolic murmur and cardiac pathology potentially associated with hemodynamic instability and pulmonary edema. Results showed SAM and incomplete coaptation of the MV (Fig. 2). And the left ventricular (LV) cavity was small (LV end-diastolic dimension: 39 mm) with notable hyperdynamic LV contraction (LV ejection fraction: 78%), suggesting intracardiac volume depletion. Although severe asymmetrical or concentric LV wall hypertrophy was not evident, relative wall thickness was 0.46 and LV mass index was 94 gm/m2, and those values corresponded to concentric remodeling of the LV (Fig. 2). Color Doppler evaluation showed significant eccentric MR caused by SAM of the MV (vena contracta width: 0.8 cm, effective regurgitant orifice: 0.6 cm2 and regurgitant volume: 60 mL, calculated with proximal isovelocity surface area method); however, MV itself was otherwise normal (Fig. 2). Continuous Doppler study of the LVOT showed a late-peaking profile of the flow with a maximal pressure gradient of 119 mm Hg estimated by systolic blood flow velocity, and it was suggestive of significant dynamic LVOT obstruction (Fig. 2). Meanwhile, early diastolic mitral inflow velocity (E) was 0.9 m/s and early diastolic mitral annulus velocity (E') at the septal corner was 0.04 m/s (E/E' ratio: 23). Inferior vena cava diameter was 1.1 cm and also showed good collapsibility (respiratory variability was above 50%).
Despite pulmonary edema on the chest radiography, we decided to restore the patient's systemic volume, because the cause of pulmonary congestion was not due to absolute systemic volume overload, but due to intracardiac volume maldistribution resulting from SAM of the MV in the volume depleted heart. After receiving fluid therapy with crystalloid and other nutritional support for five days, the patient's condition stabilized and laboratory findings gradually returned to normal. Echocardiographic evaluation performed after treatment revealed complete resolution of SAM of the MV and disappearance of the preexisting dynamic LVOT obstruction and significant MR (Fig. 3). At the same time, E/E' ratio was decreased to 13, indicating reduced left atrial (LA) pressure compared to previous study. On hospital day 38, the patient was discharged in good condition.
Our patient suffered transient heart failure with hemodynamic deterioration due to the unexpected cause of profound dehydration; therefore, the present case is clinically meaningful and provides helpful insights into intracardiac hemodynamics in a volume-depleted heart. Newly developed pulmonary congestion was suspected on the chest radiography, however, it was counterintuitive, since she was extremely dehydrated. Dehydration itself is hardly regarded as a postulated cause of cardiac failure and pulmonary edema. Interestingly, however, acute heart failure with hemodynamic impairment was paradoxically precipitated by profound dehydration in this case. In addition, careless administration of diuretics, vasodilators or catecholamine, which is mainstay of treatment of heart failure, might have been a critical mistake in this intriguing clinical setting.
Those unusual manifestations were successfully explained by echocardiography, which revealed SAM of the MV, resultant dynamic LVOT obstruction and significant MR. Dynamic LVOT obstruction with/without MR, which is often recognized in critically ill patients with or even without structural heart disease, is potentially fatal.6 The majority of patients with dynamic LVOT obstruction without organic heart disease have a history of hypertension with some degree of concentric hypertrophy and precipitating factors, such as catecholamine administration, sepsis, blood loss or dehydration, etc.4-6 In the current case, profound dehydration and anemia precipitated intraventricular obstruction and significant MR, thereby inducing an acute drop in cardiac output along with hemodynamic impairment. The patient's medical history of old cerebrovascular accident might have affected thirst and satiety mechanism of the patient7 providing a plausible explanation for the patient's extreme dehydration.
Classically, the cardinal features of cardiogenic shock include increased pulmonary capillary wedge pressure and decreased cardiac output, whereas the characteristics of hypovolemic shock include low pulmonary capillary wedge pressure and reduced cardiac output.8 Due to differences in management strategies, distinguishing between cardiogenic and hypovolemic shock is critical. In the present case, pulmonary congestion was notable and it was considered to be a consequence of elevation of pulmonary capillary pressure due to increased LA pressure which resulted from intracardiac dynamic obstruction and significant MR. Therefore, the cause of the patient's hypotension was cardiogenic rather than hypovolemic, and early recognition of the unexpected hemodynamic feature was essential for legitimate treatment. In this point of view, echocardiography was an invaluable tool in our case to define the etiology of the shock and to determine the proper management plan.
Figures and Tables
Fig. 1
(A) Chest radiograph taken previously. (B) Chest radiograph showing newly developed pulmonary congestion (arrow heads) and fluid collection in the fissure (arrow) at the time of presentation.
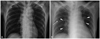
Fig. 2
(A) Two-dimensional transthoracic echocardiography shows systolic anterior motion of the mitral leaflets (arrow) with left ventricular outflow tract obstruction. (B) Color Doppler image reveals flow acceleration at the left ventricular outflow tract (white arrow) and significant mitral regurgitation (yellow arrow). (C) M-mode image shows systolic anterior motion of the mitral valve leaflets (arrow). (D) M-mode image at the midventricular cavity shows small left ventricular cavity and normal wall thickness.
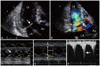
Fig. 3
(A) Two-dimensional transthoracic echocardiography reveals disappearance of systolic anterior motion of mitral leaflets (arrow) after the patient's condition had been stabilized. (B) Color flow image shows resolution of flow acceleration and normal lamina flow at the left ventricular outflow tract. Concurrently, this color flow image after volume restoration therapy shows that previously observed mitral regurgitation has been completely abolished. LA, left atrium; LV, left ventricle; Ao, ascending aorta.

References
1. Haley JH, Sinak LJ, Tajik AJ, Ommen SR, Oh JK. Dynamic left ventricular outflow tract obstruction in acute coronary syndromes: an important cause of new systolic murmur and cardiogenic shock. Mayo Clin Proc. 1999; 74:901–906.

2. Aurigemma G, Battista S, Orsinelli D, Sweeney A, Pape L, Cuénoud H. Abnormal left ventricular intracavitary flow acceleration in patients undergoing aortic valve replacement for aortic stenosis. A marker for high postoperative morbidity and mortality. Circulation. 1992; 86:926–936.

3. Villareal RP, Achari A, Wilansky S, Wilson JM. Anteroapical stunning and left ventricular outflow tract obstruction. Mayo Clin Proc. 2001; 76:79–83.

4. Mingo S, Benedicto A, Jimenez MC, Pérez MA, Montero M. Dynamic left ventricular outflow tract obstruction secondary to catecholamine excess in a normal ventricle. Int J Cardiol. 2006; 112:393–396.

5. Yang JH, Park SW, Yang JH, Cho SW, Kim HS, Choi KA, et al. Dynamic left ventricular outflow tract obstruction without basal septal hypertrophy, caused by catecholamine therapy and volume depletion. Korean J Intern Med. 2008; 23:106–109.

6. Chockalingam A, Dorairajan S, Bhalla M, Dellsperger KC. Unexplained hypotension: the spectrum of dynamic left ventricular outflow tract obstruction in critical care settings. Crit Care Med. 2009; 37:729–734.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download