Abstract
The effective and toxic ranges of anticancer drugs are very narrow and, in some cases, inverted. Thus determination of the most appropriate dosage and schedule of administration is crucial for optimal chemotherapy. In common arm trials conducted in Japan and by Southwest Oncology Group (SWOG) that used the same doses and schedules for the administration of carboplatin plus paclitaxel, the frequency of hematological toxicity was significantly higher in the Japanese trials than in the SWOG trial, despite demonstrating similar response rates. The frequency of epidermal growth factor receptor (EGFR) mutations in tumors was significantly higher among East Asian populations, and these populations are also reported to demonstrate a higher response rates to epidermal growth factor receptor tyrosine-kinase inhibitors (EGFR-TKIs). The prevalence of interstitial lung disease induced by treatment with EGFR-TKIs has been shown to be quite high in the Japanese population. Clinical trials of cetuximab against non-small cell lung cancer and of bevacizumab against stomach cancer have shown that these agents are only active in Caucasians. In a trial examining the use of sorafenib after transarterial chemoembolization in Korean and Japanese patients with advanced hepatocellular carcinoma, the compliance and dose intensity of the drug were quite low compared with other trials. Although not only identified pharmacogenomics differences but also differences in social environment, and regional medical care, including pharmacoeconomics strongly influence ethnic differences in treatment response, further identification and understanding of the pharmacogenomics underlying ethnic differences will be essential to timely and reliable global development of new anticancer drugs.
The pharmacokinetics (PK) and pharmacodynamics (PD) of most drugs can vary widely even if the same doses are administered. Many factors influence this variability.1-3 Among these clinical pharmacoethnic differences arising from functional variants in implicated genes have been reported for in 5-fluorouracil,4-6 doxorubicin,7 cyclophosphamide,8 vincristine9 and irinotecan hydrochloride.10,11 Pharmacogenomic difference in metabolizing enzymes and transporters can influence the toxicities of anticancer drugs differently across ethnic groups. Single nucleotide polymorphisms (SNPs) in UDP glucuronosyl transferase 1A1 and dihydropyrimidine dehydrogenase, which are germ line variations, have been shown to be associated with the hematological toxicities of irinotecan hydrochlolide and fluorinated pyrimidines, respectively.4,11 On the other hand, antitumor activity is mainly influenced by somatic changes which reflect the pharmacogenomics of target molecules in tumors. Even prior to epidermal growth factor receptor tyrosine-kinase inhibitors (EGFR-TKIs) development (Fig. 1), ethnic differences were reported in regard to survival outcomes between Asians and Caucasians with advanced non-small cell lung cancer (NSCLC) treated with systemic chemotherapy.12 Similarly, treatment response to EGFR-TKIs which are active against driver mutation positive NSCLC exhibited clear ethnic differences in Caucasians and East Asians due to the difference in EGFR mutation rates.13 In trials of antiEGFR and anti-vascular endothelial growth factor antibodies against lung and stomach cancer, respectively, as well as sorafenib against liver cancer, pharmacoethnic differences induced by differences in environmental and local medical practices have also been noted in addition to differences in pharmacogenomics (Table 1). Accordingly, recent phase I study on tivantinib, a MET-TKI inhibitor, showed the presence of CYP2C19 polymorphisms in 30% of Japanese patients, which was 10 times higher than that in Caucasians. In the present review, the development of protocols for global trials and interpretation of the results of such trials will be discussed from the perspective of pharmacoethnicity.
In previous common arm trials cooperatively conducted together with SWOG, differences in the effectiveness and toxicities of paclitaxel and carboplatin in NSCLC14 and of etoposide or irinotecan plus cisplatin in small cell lung cancer (SCLC) were analyzed.15 Prior to the initiation of the study, whether studies in one country could be directly extrapolated to patients within another country was unclear. The purpose of the trial was to demonstrate similarities and differences in the patient characteristics and outcomes in Japan and the United States under the same treatment regimen. The trials also sought to provide a basis for the standardization of future study design, for which to better conduct future trials in both countries.
For NSCLC, three trials were performed. Two of the studies were performed in Japan [Four Arm Cooperative Study (FACS), n=14516 and JMTO LC-00-03 study, n=19717], and one study was performed in the USA (SWOG S0003 study, n=186).18 All three studies used identical eligibility criteria, staging criteria and treatment protocols (Table 2). Paclitaxel and carboplatin were considered to be a common arm. The dose of carboplatin administered in all of the studies was an area under the curve (AUC) 6 ug/mL×min, and while the administered dose of paclitaxel was 225 mg/m2 in the JMTO and SWOG trials and 200 mg/m2 in the FACS trial. This regimen was administered every 3-4 weeks. The patient characteristics such as age, sex, proportion with stage IIIB/IV, and histology were evenly distributed in all three studies. Treatment compliance was also the same in all three studies. The response rates were 32%, 31% and 37% in the FACS, LC00-03 and S0003 studies, respectively. The median survival and the one year survival rates were higher in Japanese patients (Table 3). In addition the frequency of hematological toxicities such as grade IV neutropenia and febrile neutropenia were significantly higher among the Japanese patients (Table 4). Genotype profiles in Japanese and American patients showed differences in frequencies of variants in CYP3A4, CYP3A5, CYP2C8 and ERCC2. Among them CYP3A4 and ERCC2 were correlated with progression free survival (PFS) and treatment response, respectively. These results suggest that global clinical trials, especially those conducted internationally, should be carefully designed and conducted to account for potential genetic differences in the respective patient population.
For SCLC, the JCOG951119 and SWOG-021420 trials were conducted. Both studies compared etoposide/cisplatin vs. irinotecan/cisplatin. The dose and administration schedules of the drugs were exactly the same. The patient demographics showed that 86% and 57% of the patients in the JCOG 9511 and SWOG-0124 trials were male, respectively, and the performance status (PS) of the patients was better in the SWOG trial. Treatment delivery and dose intensities were similar for both trials. The response rates to both regimens were significantly higher in the JCOG9511 trial. The overall survival and one year survival rates were similar between the two trials for the cisplatin/etoposide regimen, however, these outcomes were significantly better for the cisplatin/irinotecan regimen in the JCOG 9511 trial (overall survival (OS): 12.8 vs. 6.8 months, one-year survival: 58% vs. 41%). On the other hand, the frequencies of hematological toxicities (>grade 3) such as neutropenia, leukopenia and anemia were significantly higher for both regimens in the JCOG 9511 trial than those in the SWOG-0124 trial. These results suggested the need for 1) consideration of different patient characteristics and outcomes amongst patients receiving identical therapy. 2) The utilization of a common arm model in prospective trials and 3) the inclusion of pharmacogenomics correlates in cancer trials where pharmacoethnic differences in drug disposition are expected.
The molecular target of EGFR-TKI was thought to be wild type EGFR during the initial development of EGFR-TKIs. During a phase I trial conducted in Japan, 5 patients with heavily treated NSCLC experienced a partial response21 despite numerous reports of a lack of a response in other Phase 1 studies except for Ranson's report.22-24 Phase II studies of gefitinib such as the Ideal 1 and 2 trials reported response rates for Japanese and Non-Japanese patients of 30 and 10%, respectively.25,26 The response rate of another EGFR-TKI, erlotinib, was also about 30% in Japanese population.27 Various clinical trials have also suggested that EGFR-TKIs are active in non-smoking East-Asian, female with adenocarcinoma whereas the BR-21 study demonstrated the completely opposite results (Fig. 2).28,29 The majority of clinical trials against unselected populations produced negative results. In first line NSCLC chemotherapy studies such as the Intact-1/-2, Talent and Tribute trials which included more than 1000 patients each, the addition of gefitinib or erlotinib did not improve overall survival.30-33 In second line trials performed in unselected patients, the adenocarISEL trial using gefitinib produced negative results34 while the BR-21 trial using erlotinib produced positive results.29 If the ISEL population was subdivided into Asians and Caucasians, gefitinib was shown to only be effective against Asian patients (Fig. 3).34 On the other hands in the BR-21 study, erlotinib was more effective against squamous carcinoma, Caucasians, and male smokers29 although recent trials examining erlotinib have reported conflicting results. (Fig. 4). EGFR mutations were first discovered in 2004, and somatic mutations in tyrosine kinase (TK) domain of EGFR were shown to be strongly associated with responses to treatment with EGFR-TKIs.35,36 Moreover the mutation rate of EGFR in patients with NSCLC was shown to be remarkably different between East Asians (35-40%) and Caucasians (<10%) (Fig. 5).37,38 Seven phase II studies on the use of EGFR-TKIs against patients with EGFR mutation demonstrated high response rates ranging from 63% to 91% across Asia. The Iressa Pan-Aisa Study (IPASS) trial, which compared gefitinib vs. carboplatin/paclitaxel in clinically selected patients, included patients from 9 Asian countries including China, Japan, Hong Kong, the Philippines, Singapore, Taiwan and Thailand, accruing 1217 patients within 18 months.39 Patient selection in the IPASS trial was, however, performed based solely on clinical factors such as adenocarcinoma and never/light smokers in addition to an East Asian ethnicity. The overall data was difficult to explain because the PFS curves crossed at 6 months. If the patients were subdivided according to the EGFR mutational status, the PFS of the gefitinib was better in the mutation positive patients, while that of the carboplatin/paclitaxel group was better in the mutation negative patients. In their study, however, EGFR mutation status was only analyzed in 36% of the patients. Substantiating their findings, the Korean First Signal Study obtained similar results. At the same time two randomized controlled trials (RCTs) using gefitinib to treat EGFR mutation positive patients were conducted by two groups in Japan, North East Japan (NEJ) 00240 and West Japan Thoracic Oncology Group (WJTOG) 3405.41 Later two RCTs using erlotinib were conducted in China (OPTIMAL)42 and Europe (EURTAC).43 All four of these trials produced similar results (Table 5), suggesting that the biological character of EGFR mutated NSCLC was similar in East Asians and Caucasians despite differences in mutation frequencies. Presently, clinical trials of next generation EGFR-TKIs are on-going. The majority of patients accrued to those trials are East Asian patients, since all of these trials required pharmacogenomic selection; for example 70% of patients in the LUX-LUNG3 global trial for afatinib are East Asian (Fig. 6).44 Fig. 7 shows the ethnic differences in the molecular classification of adenocarcinoma of the lung based on data from the National Cancer Center Research Institute of Japan and the Memorial Sloan Kettering Cancer Center-in the United States (Fig. 7). Careful selection is becoming mandatory to ensure that clinical trials are completed very quickly and yield scientifically sound data.
It is worth noting that driver mutations are likely to become the most important and promising molecular target for future anticancer drugs.45 Accordingly, possible ethnic difference in driver mutations must be kept in mind when performing preclinical and early clinical trials of molecular targeted drugs.
Interstitial lung disease (ILD) affects the lung parenchyma and alveoli of the lungs. Drug-induced ILD presents with acute diffuse alveolar damage and sometimes involves a fatal outcomes. Unfortunately no specific treatment for ILD exists. Palliative therapies with oxygen inhalation and corticosteroid administration have been attempted, but some patients require assisted ventilation and die. ILD can reportedly be induced by cytotoxic chemotherapy and radiotherapy, the prevalence of which is higher among Japanese than among Caucasians.46 EGFR-TKI-induced ILD is considered to be relatively rare47 and appears to be limited to patients in Japan.48 A WJTOG retrospective study of 1976 patients reported the frequencies of ILD and ILD-induced mortality by gefitinib as 3.2 and 1.3% respectively. The risk factors thereof were a male gender, a history of smoking and a history of pulmonary fibrosis.28 AstraZeneca performed a prospective study of 3322 patients receiving gefitinib. ILD and ILD-induced death were observed in 5.8 and 2.5% of these patients, respectively. The reported risk factors were a history of smoking, a poor PS, a history of pulmonary fibrosis and prior chemotherapy. In a nested case controlled study performed by AstrasZeneca, the overall odds ratio for ILD due to gefitinib treatment, compared with chemotherapy, was 3.2.49 This specific trial showed that, among the patients who died as a result of ILD, 31.6% had received gefitinib and 27.9% undergone chemotherapy, respectively. Based on the AstraZeneca global Drug Safety Database, the reported rate of ILD-type events in patients receiving gefitinib was only 0.23% throughout the rest of the world, excluding Japan, according to the records of more than 215000 patients treated with gefitinib. Even in other East Asian countries such as Korea and Taiwan the rate was only 0.17%. On the other hand the percentages of patients involving a fatal outcome after developing ILD were similar, 37% in Japan and 31% in other countries. Within the case-controlled study performed by AstraZeneca, analyses were conducted to identify genetic and proteomic predictors for ILD, unfortunately however, no specific biomarkers capable of predicting ILD have been identified.
The cetuximab plus chemotherapy in patients with advanced non-small cell lung cancer (FLEX) trial consisted of 1125 patients and was designed to demonstrate superior overall survival for cetuximab when used in combination with chemotherapy compared with chemotherapy alone as a first-line therapy for patients with advanced NSCLC. As the chemotherapeutic regimen, cisplatin and vinorelbine were selected. Cetuximab, at an initial dose of 400 mg/m2 followed by 250 mg/m2 weekly, was given concurrently with chemotherapy and as a maintenance regimen in the cetuximab group. Overall survival (10.1 vs. 11.3 months), response rate (29% vs. 36%) and the time to treatment failure (4.2 vs. 3.7 months) were significantly better in the cetuximab group than chemotherapy alone group although the PFS was 4.8 months in both groups (Table 6). The study included 946 Caucasians and 121 Asians. The median survival periods for the Caucasians and Asians were 9.6 and 19.5 months, respectively. Among the Asian patients, adenocarcinoma (44% vs. 72%), a female gender (27% vs. 46%), never smokers (17% vs. 52%), good ECOG PS (81% vs. 94%) were dominant (Table 7). Most of the Asian patients received treatment with an EGFR-TKI (17% vs. 61%) after the first line chemotherapy. No statistically significant difference in OS (17.6 vs. 20.4 months), or RR (50% vs. 44%) was seen between the chemotherapy+cetuximab and the chemotherapy alone group in the Asian population (Table 8). However, in Caucasians, chemotherapy+cetuximab produced a significantly better survival period, compared with chemotherapy alone. These discrepancies can be explained by differences in 1) the patients's backgrounds such as histology, gender, smoking status and PS as well as 2) treatment with a second/third line EGFR-TKI. Additionally, differences in EGFR mutation rate, of course, may have also influenced the longer survival in Asian patients. The FLEX study effectively demonstrated the difficulty of interpreting global trials in which pharmacoethnic differences are present among the patient population.
The AVAGAST trial was conducted as a randomized double-blinded placebo-controlled phase III trial to compare capecitabine/cisplatin+bevacizumab vs. chemotherapy alone in patients with locally advanced or metastatic gastric cancer. The study stratified patients according to geographic region in addition to fluoropyrimidine backbone and disease status. A total of 774 patients were included in the study. Patients were evenly distributed to two arms according to gender, age, ECOG PS, disease sites such as the stomach fundus and the gastro-esophageal junction, disease measurability, metastatic sites, prior gastrectomy, liver metastases and the three stratification factors mentioned above. In both groups, nearly half of the patients were from Asian countries. The percentage of patients with an intestinal histology was slightly higher in the bevacizumab group (40% vs. 35%) (Table 9). Overall survival was not statistically significantly different between the two groups (p=0.1002). PFS was significantly better in the bevacizumab group (p=0.0037, 6.7 months vs. 5.3 months). The regional differences in efficacy are shown in the Fig. 8. OS and PFS were longer in the Asian population compared with the European and Pan American populations in both groups. The Hazard ratios (HRs) of OS for Pan Americans and those of PFS for Europeans and Pan Americans were smaller than 1.0 suggesting that the addition of bevacizumab to these populations may have contributed to prolonged survival (Fig. 8). No notable differences in patient characteristics were seen according to the region. Among Asians, the most common primary legion was the stomach fundus (94%), almost all the patients had distant metastases (99%), more patients had undergone prior gastrectomy (32%) and fewer patients had liver metastases (27%) (Table 9). The majority of the Asian patients (248/376: 66%) received second line chemotherapy. On the other hand only 31% (78/249) and 21% (32/149) of the European and Pan-American patients received second line chemotherapy, respectively (Table 10). The AVAGAST trial did not meet the primary endpoint for OS. Increase in secondary endpoints (PFS and best RR) suggested some biological activity of bevacizumab against advanced gastric cancer. The heterogenous efficacy results in both treatment arms across geographic regions may have been caused by differences in the tumor burden, tumor status, medical care and genetics. This study might have also encountered patients with different pharmacoethnicity.
The activity of sorafenib, a multikinase inhibitor was shown to lead to a prolonged OS (10.7 vs. 7.9 months: HR=0.69) and median time to radiologic progression (5.5 vs. 2.8 months) in patients with advanced hepatocellular carcinoma in the Sorafenib HCC Assessment Randomized Protocol trial which consisted of 602 patients who had not undergone prior systemic therapy.55 In another clinical trial that included patients from the Asia-Pacific region, sorafenib was found to prolong OS (6.5 vs. 4.2 months: HR=0.68) and time to progression (2.8 vs. 1.4 months: HR=0.57).56 In Japanese and Korean patients, an additional phase III trial on sorafenib was conducted after trans-arterial chemo-embolization (TACE).57 In both countries TACE has practically been used for the treatment of advanced hepatocellular carcinoma. The trial showed that sorafenib did not significantly prolong TTP in patients who responded to TACE. Several factors may have contributed to these discrepant results. High percentages of patients receiving sorafenib required dose reductions (73%) and/or treatment interruptions (91%). Accordingly the median daily dose (386 mg) was significantly lower than the planned dose. Twenty-six percent and 44% of the patients in the SHARP trial as well as 31% and 43% of the patients in the sorafenib Asia-Pacific (AP) trial required dose reductions and interruptions, respectively. The median daily doses of sorafenib were 797 and 795 mg in the SHARP and AP trials, respectively. In the sorafenib after TACE trial conducted in Korea and Japan, outcomes in Korean patients were better than that in Japanese patients and sorafenib prolonged TTP in Korean patients (HR=0.38) based on the results of subset analyses. However, some differences were observed in baseline characteristics between Korean and Japanese patients. The Japanese patients were older, and a higher percentage presented with >3 lesions at the time of enrollment in the study. The Japanese patients were also less likely to have received >one TACE to achieve complete response prior to treatment with sorafenib. These subgroups also differed in the principle etiology of hepatocellular carcinoma, as more than 70% of the Japanese patients were infected with hepatitis C virus and more than 70% of Korean patients were infected with hepatitis B virus. These results suggest that major pharmacoethnic differences exist even between Korean and Japanese patients.
Ethnic diversity is recognized as an important factor accounting for inter-individual variations in anticancer drug responsiveness and toxicity. However, similar doses of anticancer drugs have been prescribed to different ethnic populations without consideration of the potential differences in pharmacokinetics and pharmacodynamics, both of which are essentially influenced by pharmacogenomics. Although pharmacoethnicity is determined by genetic and non-genetic factors, the latter factors have not yet been well identified. The possible determinants of ethnicity could include 1) environmental factors that influence bioavailability and metabolism, such as the frequency of smoking, alcohol drinking, herbal medicine use and local dietary varieties, 2) local medical care preferences, 3) ethnic specific drug-drug interactions influenced by drug lag in a specific region, 4) variability of genetic polymorphisms in drug metabolizing enzymes and transporters, and 5) the prevalence of an ethnically restricted mutations in a drug's receptor/target that may cause a particular sensitivity or resistance to that drug.1
Studies on pharmacoethnicity face many challenging issues. However, preclinical and clinical studies should be carefully designed to account for these problems. 1) Clinical trials on pharmacoethnicity require diverse populations. International collaboration, including global trials, is mandatory and repeated trials using the same treatment strategies should be conducted in multiple countries. 2) Chemotherapy-related effects are likely to be under multi-gene control. Unbiased genome-wide models are needed and such models should include multiple mechanism-related and metabolism-related pathways. 3) Potentially important polymorphisms or mutations are generally uncommon. The sample size of clinical trials should be sufficient to enable an appropriate statistical power. Clinical trials in ethnic populations with a specific phenotype of interest may sometimes be necessary. To discover rare variants, new generation of sequencing methodology should be introduced. 4) The detection of genome-wide associations requires multiple SNP testing, which may generate several false-positive SNPs. Accordingly, test and validation sets are mandatory. In addition, both of preclinical and clinical validations, as well as the application of a rigorous statistical methodology is needed. Furthermore, as 5) anti-cancer drugs cannot be administered to healthy volunteers, in vitro cell based models are needed1 and pharmacogenomical selection of patients is recommended by appropriate biomarkers.
The clinical trials included in the present review were large enough to identify ethnic differences, despite the numerous hidden factors that remain unknown. As shown by common arm trials between USA and Japan, Asian patients experience more frequent and profound neutropenia despite receiving the same treatment doses, schedules and same pharmacokinetics. A difference in sensitivity at receptor site has been suggested to be the main cause of these observations, but a concrete mechanism, thereof has not been clarified. On the other hand, the survival period of NSCLC patients was significantly longer among Asian patients than among Caucasians even before EGFR-TKIs became available. In a common arm trial, the OS and one-year survival were both significantly better in Asian patients although response rates were exactly the same. Differences in sensitivity to EGFR-TKI have been clearly explained by EGFR mutation in both Asians and Caucasians. However, why the frequency of EGFR mutation is higher among Asian patients remains unknown. Some germ line and environmental factors may influence this mutation rate. However, all previous genome-wide and proteome analyses that have been performed in association with prospective clinical trials failed to pick up on relevant factors. There is also no clear explanation as to why EGFR-TKI-induced ILD is observed so frequently in Japanese patients only. To date, only clinical characteristics indicating a susceptibility to ILD have been identified. The data of three trials the FLEX, AVAGAST, and Sorafenib after TACE trials, suggested that disease status and local medical care preference strongly influenced the OS of the included patients.
Recently the Shizuoka Cancer Center reported interesting results on Phase I study of ARQ197, a selective, non-ATP competitive inhibitor of c-MET, a receptor tyrosine kinase involved in tumor migration, invasion and proliferation (Fig. 9). Therein, the ratios of poor metabolizers (PM), who exhibited a single nucleotide polymorphism in CYP2C9, a major metabolizing enzyme for ARQ197, among Caucasians and Asians have been reported to be 3 and 20%, respectively (Table 11). Recommended phase II dose of ARQ197 for subjects of western countries has been decided to be a single dose of 360 mg bid. On the other hand, the study in Japan demonstrated that CYP2C19 genotype clearly affected exposures such as AUC and C-max of ARQ, which led to the designation of two different recommended doses for phase II trials, 360 mg bid for extensive metabolizer patients and 240 mg bid for PM patients (Fig. 10). Clear ethnic differences mandates the necessity of different protocols for phase II studies of Asians and Caucasians.58
In summary basic pharmacogenomic differences could only be identified from the prospective analyses of patients of a homogeneous background. The clarification of pharmacoethnic differences will be crucially important to the future development of new anticancer drugs.
Figures and Tables
Fig. 1
Ethnic differences in survival outcome of NSCLC. A meta-analysis of randomized trials. Asian: 91 studies, Caucasian: 301 studies. Survival was better in Asian that Caucasian.12 EGFR-TKI, epidermal growth factor receptor tyrosine-kinase inhibitor; NSCLC, non-small cell lung cancer; CDDP, cisplatin.

Fig. 2
EGFR-TKI is effective in patients with distinct clinico-pathological and pharmacogenomical features. EGFR, epidermal growth factor receptor; TKI, tyrosine-kinase inhibitor; BAC, bronchial alveolar cell; FISH, fluorescence in situ hybrydization.

Fig. 3
ISEL study comparing gefitinib and placebo showed survival beneft only in Asian subset.34 MST, median survival time; ISEL, Iressa survival evaluation in lung cancer.

Fig. 5
Significantly high EGFR mutation rate in Asian NSCLC patients. EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer.

Fig. 6
Global Ist line clinical trials of Afatinib vs. CT for molecularly-selected NSCLC patients (LUX-LUNG3). EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer.
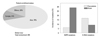
Fig. 7
Ethnic difference for molecular classification of adenocarcima. EGFR, epidermal growth factor receptor.

Fig. 10
Phase I combination study with erlotinib in Japan. EM, extensive metabolizer; PM, poor metabolizer.

Table 1
Factors Influencing on Pharmacoethnicity: Ethnic Diversity in Anticancer Effect and Toxicity

Table 6
Response Rate, Progression-Free Survival and Time to Treatment Failure in Whole Population of FLEX Trial (CDDP+VNB w/wo Cetuximub)

Table 7
Differences in Prognostic Factors, Post-Study Treatment and OS between Caucasians and Asians in FLEX trial (CDDP+VNB w/wo Cetuximab)

ACKNOWLEDGEMENTS
This paper was presented at the 6th Japan-US Cancer Therapy International Joint Symposium in Hiroshima on July19-21, 2012.
References
1. O'Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res. 2009. 15:4806–4814.
2. Huang RS, Ratain MJ. Pharmacogenetics and pharmacogenomics of anticancer agents. CA Cancer J Clin. 2009. 59:42–55.

3. Huang SM, Temple R. Is this the drug or dose for you? Impact and consideration of ethnic factors in global drug development, regulatory review, and clinical practice. Clin Pharmacol Ther. 2008. 84:287–294.

4. Schwab M, Zanger UM, Marx C, Schaeffeler E, Klein K, Dippon J, et al. Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: a prospective clinical trial by the German 5-FU Toxicity Study Group. J Clin Oncol. 2008. 26:2131–2138.

5. Lecomte T, Ferraz JM, Zinzindohoué F, Loriot MA, Tregouet DA, Landi B, et al. Thymidylate synthase gene polymorphism predicts toxicity in colorectal cancer patients receiving 5-fluorouracil-based chemotherapy. Clin Cancer Res. 2004. 10:5880–5888.

6. Marsh S, Collie-Duguid ES, Li T, Liu X, McLeod HL. Ethnic variation in the thymidylate synthase enhancer region polymorphism among Caucasian and Asian populations. Genomics. 1999. 58:310–312.

7. Lal S, Wong ZW, Jada SR, Xiang X, Chen Shu X, Ang PC, et al. Novel SLC22A16 polymorphisms and influence on doxorubicin pharmacokinetics in Asian breast cancer patients. Pharmacogenomics. 2007. 8:567–575.

8. Petros WP, Hopkins PJ, Spruill S, Broadwater G, Vredenburgh JJ, Colvin OM, et al. Associations between drug metabolism genotype, chemotherapy pharmacokinetics, and overall survival in patients with breast cancer. J Clin Oncol. 2005. 23:6117–6125.

9. Renbarger JL, McCammack KC, Rouse CE, Hall SD. Effect of race on vincristine-associated neurotoxicity in pediatric acute lymphoblastic leukemia patients. Pediatr Blood Cancer. 2008. 50:769–771.

10. Minami H, Sai K, Saeki M, Saito Y, Ozawa S, Suzuki K, et al. Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet Genomics. 2007. 17:497–504.

11. Innocenti F, Kroetz DL, Schuetz E, Dolan ME, Ramírez J, Relling M, et al. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol. 2009. 27:2604–2614.

12. Soo RA, Loh M, Mok TS, Ou SH, Cho BC, Yeo WL, et al. Ethnic differences in survival outcome in patients with advanced stage non-small cell lung cancer: results of a meta-analysis of randomized controlled trials. J Thorac Oncol. 2011. 6:1030–1038.

13. Toyooka S, Kiura K, Mitsudomi T. EGFR mutation and response of lung cancer to gefitinib. N Engl J Med. 2005. 352:2136.
14. Gandara DR, Kawaguchi T, Crowley J, Moon J, Furuse K, Kawahara M, et al. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol. 2009. 27:3540–3546.

15. Lara PN Jr, Chansky K, Shibata T, Fukuda H, Tamura T, Crowley J, et al. Common arm comparative outcomes analysis of phase 3 trials of cisplatin + irinotecan versus cisplatin + etoposide in extensive stage small cell lung cancer: final patient-level results from Japan Clinical Oncology Group 9511 and Southwest Oncology Group 0124. Cancer. 2010. 116:5710–5715.

16. Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007. 18:317–323.

17. Kubota K, Kawahara M, Ogawara M, Nishiwaki Y, Komuta K, Minato K, et al. Vinorelbine plus gemcitabine followed by docetaxel versus carboplatin plus paclitaxel in patients with advanced non-small-cell lung cancer: a randomised, open-label, phase III study. Lancet Oncol. 2008. 9:1135–1142.

18. Williamson SK, Crowley JJ, Lara PN Jr, McCoy J, Lau DH, Tucker RW, et al. Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: Southwest Oncology Group Trial S0003. J Clin Oncol. 2005. 23:9097–9104.

19. Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002. 346:85–91.

20. Lara PN Jr, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009. 27:2530–2535.

21. Nakagawa K, Tamura T, Negoro S, Kudoh S, Yamamoto N, Yamamoto N, et al. Phase I pharmacokinetic trial of the selective oral epidermal growth factor receptor tyrosine kinase inhibitor gefitinib ('Iressa', ZD1839) in Japanese patients with solid malignant tumors. Ann Oncol. 2003. 14:922–930.

22. Baselga J, Rischin D, Ranson M, Calvert H, Raymond E, Kieback DG, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002. 20:4292–4302.

23. Herbst RS, Maddox AM, Rothenberg ML, Small EJ, Rubin EH, Baselga J, et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol. 2002. 20:3815–3825.

24. Ranson M, Hammond LA, Ferry D, Kris M, Tullo A, Murray PI, et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol. 2002. 20:2240–2250.

25. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003. 21:2237–2246.

26. Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003. 290:2149–2158.

27. Takahashi T, Yamamoto N, Nukiwa T, Mori K, Tsuboi M, Horai T, et al. Phase II study of erlotinib in Japanese patients with advanced non-small cell lung cancer. Anticancer Res. 2010. 30:557–563.
28. Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006. 24:2549–2556.

29. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005. 353:123–132.

30. Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004. 22:777–784.

31. Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol. 2004. 22:785–794.
32. Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005. 23:5892–5899.

33. Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007. 25:1545–1552.

34. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005. 366:1527–1537.

35. Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004. 304:1497–1500.

36. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004. 350:2129–2139.

37. Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, Mori S, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005. 23:2513–2520.

38. Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005. 23:6829–6837.

39. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009. 361:947–957.

40. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010. 362:2380–2388.

41. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010. 11:121–128.

42. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011. 12:735–742.

43. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012. 13:239–246.
44. Yang JCH, Schuler MH, Yamamoto N, O'Byrne KJ, Hirsh V, Mok T, et al. LUX-Lung3: a randomized, open-label phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations. ASCO 2012. J Clin Oncol. 2012. 30:suppl. Abstract LBA7500.
45. Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science. 2002. 297:63–64.
46. Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics. 2000. 20:1245–1259.

47. Kishi K, Nakata K, Yoshimura K. Efficacy of gefitinib in a patient with lung cancer associated with idiopathic pulmonary fibrosis. J Thorac Oncol. 2006. 1:733–734.

48. Takano T, Ohe Y, Kusumoto M, Tateishi U, Yamamoto S, Nokihara H, et al. Risk factors for interstitial lung disease and predictive factors for tumor response in patients with advanced non-small cell lung cancer treated with gefitinib. Lung Cancer. 2004. 45:93–104.

49. Kudoh S, Kato H, Nishiwaki Y, Fukuoka M, Nakata K, Ichinose Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. 2008. 177:1348–1357.

50. Jones SJ, Milenkova T. ILD during erlotinib and gefitinib treatment in Japanese patients with non-small cell lung cancer. J Thorac Oncol. 2010. 5:1877–1878.

51. Lind JS, Smit EF, Grünberg K, Senan S, Lagerwaard FJ. Fatal interstitial lung disease after erlotinib for non-small cell lung cancer. J Thorac Oncol. 2008. 3:1050–1053.

52. Liu V, White DA, Zakowski MF, Travis W, Kris MG, Ginsberg MS, et al. Pulmonary toxicity associated with erlotinib. Chest. 2007. 132:1042–1044.

53. Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009. 373:1525–1531.

54. Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011. 29:3968–3976.

55. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008. 359:378–390.

56. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009. 10:25–34.





 PDF
PDF ePub
ePub Citation
Citation Print
Print










 XML Download
XML Download