This article has been retracted. See "Retraction: Prognostic and Predictive Value of Carcinoembryonic Antigen and Cytokeratin-19 Fragments Levels in Advanced Non-Small Cell Lung Cancer Patients Treated with Gefitinib or Erlotinib. Yonsei Med J 2012;53:931-9." in Volume 54 on page 269.
Abstract
Purpose
The prognostic and predictive value of pretreatment serum levels of carcinoembryonic antigen (CEA) and cytokeratin-19 fragments (CYFRA 21-1) were assessed in advanced non-small cell lung cancer (NSCLC) patients treated with gefitinib or erlotinib.
Materials and Methods
Pretreatment CEA and CYFRA 21-1 were measured in 123 advanced NSCLC patients receiving gefitinib or erlotinib. High CEA levels (h-CEA) were significantly associated with females, patients with adenocarcinoma, and non-smokers.
Results
Low CYFRA 21-1 levels (l-CYFRA) were significantly associated with a good performance status (ECOG PS 0-1). The overall response rate (RR) was 27.6%, and higher RR was associated with adenocarcinoma, h-CEA, and epidermal growth factor receptor (EGFR) mutation. Patients with h-CEA had significantly longer progression-free survival (PFS) (p=0.021). Patients with l-CYFRA had significantly longer PFS and overall survival (p=0.006 and p<0.001, respectively). Of note, h-CEA and l-CYFRA had good prognosis in patients with unknown EGFR mutation status or patients with squamous cell carcinoma (p=0.021 and p=0.015, respectively). A good ECOG PS (HR=0.45, p=0.017), h-CEA (HR=0.41, p=0.007), l-CYFRA 21-1 (HR=0.52, p=0.025), and an EGFR mutation (HR=0.22, p<0.001) were independently predictive of a longer PFS.
Lung cancer is the leading cause of cancer-related mortality in the world. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer patients.1 The oral small molecule epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) such as gefitinib and erlotinib active responses in 10-18% of patients who failed on prior chemotherapy. Erlotinib has a 2-month median survival advantage over placebo,2 and gefitinib is not inferior compared with docetaxel.3
Treatment with EGFR TKI is effective in women, Asians, non-smokers, and patients with adenocarcinoma. An EGFR mutation was found to be the most important predictive factor for a response to an EGFR TKI.4 However, acquiring adequate tissue for EGFR mutational analysis is often not feasible, particularly in patients with advanced disease.2-4 Therefore, the identification of clinical parameters that can serve as surrogates for EGFR mutation may prove useful when mutational analysis is not feasible. A recent study reported that the molecular analysis of circulating tumor cells (CTCs) from the peripheral blood of patients with lung cancer was useful in monitoring changes in epithelial tumor genotypes during the course of treatment.5 However, this molecular analysis could prove to be difficult as a specific microfluidic-based device called the CTC chip is required.
A marker that is easily analyzed and predicts responses to EGFR TKI treatment is needed. Some serum markers have been considered potentially prognostic and predictive in NSCLC. Among these NSCLC markers, carcinoembryonic antigen (CEA) and cytokeratin-19 fragments (CYFRA 21-1) have been considered sensitive and valuable tumor markers for diagnosis, prognosis, and therapy monitoring.6-10 According to recent reports, CEA and CYFRA 21-1 were significant predictors of sensitivity and survival in patients treated with gefitinib.11-13 Therefore, we investigated the clinical significance of the pretreatment serum levels of CEA and CYFRA 21-1 in advanced NSCLC patients who were treated with gefitinib or erlotinib.
We retrospectively collected clinical data on NSCLC patients who had measured pretreatment levels of CEA and CYFRA 21-1 and received gefitinib or erlotinib in the Severance Hospital, Yonsei University Health System, Seoul, Korea, from January 2006 to December 2008. Variables used in the pretreatment analysis were age, gender, clinical stage, Eastern Cooperative Oncology Group (ECOG) performance status (PS), histological type, smoking history, number of prior chemotherapy regimens, and EGFR mutation if possible. Serum CEA (normal range, 0-5 ng/mL) and CYFRA 21-1 (normal range, 0-3.3 ng/mL) were measured by using a chemiluminescense enzyme immunoassay kit (Beckman Coulter, Inc., Brea, CA, USA) and an electrochemiluminescense immunoassay on an automatic analyzer (Elecsys 200; Roche Diagnostics Corp, Indianapolis, IN, USA), respectively, before TKI treatment. Histological analysis of tumors was based on the WHO classification for cell types.14 The clinical response to the drug was defined according to the response evaluation criteria of RECIST 1.0 for patients with measurable disease.15 Nucleotide sequencing of the kinase domain of EGFR (exons 18 to 21) was performed using nested polymerase chain reaction amplification of individual exons. Details of sequencing have been described previously.16 This study was approved by the institutional review board of the Yonsei University Health System (Approval No. 4-2009-0700).
The association between pretreatment levels of CEA and CYFRA 21-1 and other categorical clinical variables were compared using Pearson's chi-squared test. Progression-free survival (PFS) was defined as the time from the start day of TKI treatment until the date of tumor progression or death. Overall survival (OS) was measured from the date of diagnosis to the date of death or final follow-up. The survival data were estimated using a Kaplan-Meier curve and compared using the log-rank test. Multivariate analyses were performed to find prognostic markers using Cox's proportional hazards model. A p-value of less than 0.05 was considered statistically significant.
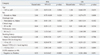
Four hundred fifty one patients were treated with erlotinib or gefitinib in Yonsei University Health System from January 2006 to December 2008, and 123 patients had measured pretreatment levels of CEA and CYFRA 21-1 and were included in this study. The clinicopathological characteristics of 123 patients are summarized in Table 1. Notably, a high serum CEA level (≥5 ng/mL) was observed in 70 patients (56.9%), and was significantly more frequent in females, patients with adenocarcinoma, and patients without a history of smoking. On the other hand, 64 patients (52%) had an elevated serum CYFRA 21-1 level (≥3.3 ng/mL), which was significantly more frequent in patients with a poor ECOG PS (p=0.017) and in those with a history of smoking (p=0.072). There was no difference in either CEA or CYFRA 21-1 levels in terms of EGFR mutation status.
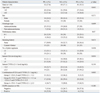
The median follow-up duration was 9.0 months (range, 0.2-43 months). The median PFS was 5.0 months [95% confidential interval (CI) 3.3-6.7 months], and the median OS was 16.0 months (95% CI, 8.7-23.3 months). Response was not assessable in 7 patients; four patients died and three patients refused treatment before response evaluation. Thirty-two of the evaluable 116 patients (27.6%) showed partial responses. The response rate to EGFR TKIs was significantly higher in patients with adenocarcinoma, an EGFR mutation, and high serum CEA levels (≥5 ng/mL). The disease control rate in the patients with high CEA levels was significantly higher than that with low CEA levels (75% vs. 51.9%, p=0.034). There were no differences in the response rates according to gender, smoking history, or the number of prior chemotherapy regimen. There was a trend towards a better response rate in patients with low CYFRA 21-1 levels (p=0.104). To evaluate whether the combination of CEA and CYFRA 21-1 levels improved the prediction accuracy, patients were divided into three groups according to their CEA and CYFRA 21-1 levels. Patients with a low CEA and a high CYFRA 21-1 level were defined as group A (CEA <5 ng/mL and CYFRA 21-1 ≥3.3 ng/mL, n=24), while those with both low or high CEA and CYFRA 21-1 levels were labeled group B (CEA <5 ng/mL and CYFR 21-1 <3.3 ng/mL, or CEA ≥5 ng/mL and CYFR 21-1 ≥3.3 ng/mL, n=66). Finally, patients with high CEA and low CYFRA 21-1 levels were defined as group C (CEA ≥5 ng/mL and CYFRA 21-1 <3.3 ng/mL, n=26). The three groups showed significantly different response rates, with the best responses in group C (42.3% vs. 25.8% vs. 16.7%, p=0.005, for groups C, B, and A, respectively) (Table 2).
Patients with high CEA levels had significantly better PFS than those with low CEA levels (7.0 months vs. 4.0 months, p=0.021). In contrast, patients with low CYFRA 21-1 levels also had significantly better PFS than those with high CYFRA 21-1 levels (8.1 months vs. 3.0 months, p=0.006). When sub-grouped by combined CEA and CYFRA 21-1 levels, the three groups showed significantly different PFS, and group C showed the longest PFS among the three groups (15.0 months vs. 4.0 months vs. 2.0 months, p<0.001, for groups C, B, and A, respectively) (Fig. 1). Especially, group C also had the longest PFS in patients with squamous cell carcinoma (Fig. 2). In addition, high CEA and low CYFRA 21-1 levels were a significant prognostic marker not only in patients with EGFR-mutant tumors, but also in patients with unknown EGFR-mutation status (Fig. 3). Finally, univariate analysis revealed several significant factors for PFS, including good ECOG PS (6.1 months vs. 3.0 months, p=0.016) and positive EGFR mutation status (11.0 months vs. 2.0 months, p<0.001) (Table 3).
Patients with good ECOG PS and a positive EGFR mutation status also had significantly longer OS than those who had a poor ECOG PS and a negative EGFR mutation status (ECOG PS, 29.6 months vs. 6.1 months, p<0.001; EGFR mutation status, 22.0 months vs. 7.1 months, p=0.038, respectively). However, OS was not different by pretreatment CEA levels. Patients with low CYFRA 21-1 levels had a longer OS than those with high CYFRA 21-1 levels (not reached vs. 8.0 months, p<0.001). Patients in group C also had the longest OS among the three groups (Table 4, Fig. 4).
Multivariate analysis using a Cox proportional hazards model indicated that a good ECOG PS, positive EGFR mutation status, high pretreatment CEA levels, and low pretreatment CYFRA 21-1 levels were independent predictive factors for PFS. Meanwhile, predictive factors for OS included a good ECOG PS, positive EGFR mutation status, and low CYFRA 21-1 levels, but not high CEA levels (Table 4).
Detection of mutation in the EGFR gene in NSCLC patients treated with EGFR TKI is the most important factor for the prediction of a good response to these drugs.4 However, the detection of an EGFR mutation is difficult due to the limited amount of available tissue.2-4 Therefore, another biomarker that can improve the prediction of response to these targeted drugs is needed.
CEA was first described by Gold and Freedman17 in 1965 as an antigen expressed by gastrointestinal carcinoma cells. Although CEA was often falsely elevated in smokers and in patients with restrictive or obstructive pulmonary disease,18-20 abnormally elevated CEA levels were reported in 30-70% of patients with NSCLC and were most frequently observed in patients with adenocarcinoma and advanced stage carcinoma.21 In addition, several studies have shown that high CEA levels were a potential marker of poor prognosis in NSCLC regardless of treatment.7,21
On the contrary, Okamoto, et al.11 reported that patients treated with EGFR TKI with high pretreatment levels of CEA had a longer survival and a better response than those with low CEA levels. They attributed this to a possible anti-apoptotic signal of the mutant EGFR pathway that may elevate the expression level of CEA protein. Our data are similar to the data of Okamoto, et al.11 Shoji, et al.22 reported that the rate of EGFR gene mutation is significantly increased as the serum CEA level increases (for serum CEA levels of <5, ≥5 but <20, and ≥20 the rate of EGFR gene mutation was 35%, 55%, and 87.5%, respectively; p=0.040). However, our data showed that the status of EGFR mutation made no difference in the CEA levels. According to previous reports, the function of CEA has remained unelucidated, and may include the following: first, CEA is a cell surface adhesion protein and may play a role in cell-to-cell adhesion;23 second, over-expression of CEA is thought to play a role in tumorigenesis;24 third, CEA has a dominant effect in blocking differentiation, and it also cooperates with Myc and Bcl-2 in cellular transformation;25 and fourth, it can inhibit cell death induced by a loss of anchorage to the extracellular matrix (anoikis).26 Although these results mean that CEA has anti-apoptotic effects in cancer cells, a direct relationship between high levels of CEA and response to EGFR TKI has not yet been established.
CYFRA 21-1, a fragment of cytokeratin subunit 19, was first identified in 1993 as a valuable marker in lung cancer patients.27 CYFRA 21-1 was found to be associated with TNM stage and ECOG PS, reflecting an unfavorable prognosis for NSCLC patients regardless of treatment.8,21,28-30 In our study, patients with poor ECOG PS had higher CYFRA 21-1 levels than those with good ECOG PS (6.4 ng/mL vs. 3.0 ng/mL, p=0.03). Furthermore, patients with high CYFRA 21-1 levels were more likely to have a history of smoking; however, this association was not significant (p=0.072). Previous studies have also reported that smoking has no effect on serum CYFRA 21-1 levels.31,32 In the present study, univariate and multivariate analyses demonstrated that CYFRA 21-1 levels higher than 3.3 ng/mL had an independent negative impact on PFS (HR=1.93, 95% CI 1.09-3.44, p=0.025) and OS (HR=2.76, 95% CI 1.38-5.53, p=0.0004). Therefore, CYFRA 21-1 seems to be an independent marker for poor prognosis in NSCLC patients receiving EGFR TKI, which is consistent with a previous study.12
We have demonstrated that pretreatment levels of CEA and CYFRA 21-1 serve as prognostic and predictive markers in NSCLC patients treated with gefitinib or erlotinib. Patients with a high pretreatment CEA level showed better responses and longer PFS, and patients with a low pretreatment CYFRA 21-1 level showed longer PFS and OS. In addition, the prediction accuracy of EGFR TKI response and prognosis improved when all patients were divided into three groups according to combined levels of CEA and CYFRA 21-1.
It is difficult to predict the high efficacy of EGFR TKIs when they are used in patients with non-adenocarcinoma histology because the rate of EGFR mutation is extremely rare in these tumors.33 However, this study revealed that CEA and CYFRA 21-1 can also be prognostic markers in patients with squamous cell carcinoma or patients with unknown EGFR mutation status (Figs. 2 and 3).
Recently, genotype-driven therapy has been attempted. The mutation of EGFR and K-ras gene, and ALK rearrangement are three major oncogenic alterations in NSCLC.34 Result from the recently presented clinical trial of crizotinib indicates that small molecule TKI targeting ALK translocation is very effective agent in ALK-positive NSCLC.35 Furthermore, according to an encouraging result from BATTLE trial, sorafenib may be used in NSCLC with KRAS mutation.36 Therefore, when we have sufficient tumor tissue and targeted agents, we could get genotype-driven therapies in NSCLC. However, the molecular marker test, such as EML4/ALK, B-raf or K-ras, could not be checked routinely in clinical practice, especially because we do not have available tumor tissues. Accordingly, when the patients do not have sufficient tumor tissues or any other predictive biomarkers, EGFR TKI could be a good treatment option in patients with high CEA and low CYFRA 21-1.
In conclusion, pretreatment serum levels of CEA and CYFRA 21-1 are simple and easy to detect, and can serve as predictive and prognostic factors for advanced NSCLC patients who are being treated with EGFR TKIs, especially in patients with squamous cell carcinoma or patients with unknown EGFR mutation status.
Figures and Tables
Fig. 1
Progression-free survival curves according to pretreatment serum levels of carcinoembryonic antigen (CEA) and cytokeratin-19 fragments (CYFRA 21-1). (A) CEA. (B) CYFRA 21-1. (C) Combinations of CEA and CYFRA 21-1 by group: a, patients with a low level of CEA and a high level of CYFRA 21-1; b, patients with both low or high level of CEA and CYFRA 21-1; and c, patients with a high level of CEA and a low level of CYFRA 21-1. PFS, progression-free survival.

Fig. 2
Progression-free survival curves according to pretreatment serum levels of combination of carcinoembryonic antigen (CEA) and cytokeratin-19 fragments (CYFRA 21-1) by histologic difference. a, Patients with a low level of CEA and a high level of CYFRA 21-1; b, patients with both low or high level of CEA and CYFRA 21-1; and c, patients with a high level of CEA and a low level of CYFRA 21-1 (A) patients with adenocarcinoma, (B) in patients with squamous cell carcinoma. PFS, progression-free survival.

Fig. 3
Progression-free survival curves according to pretreatment serum levels of combination of carcinoembryonic antigen (CEA) and cytokeratin-19 fragments (CYFRA 21-1) by epidermal growth factor receptor (EGFR) mutation status; a, patients with a low level of CEA and a high level of CYFRA 21-1; b, patients with both low or high level of CEA and CYFRA 21-1; and c, patients with a high level of CEA and a low level of CYFRA 21-1. (A) Patients with negative EGFR mutation, (B) in patients with positive EGFR mutation, (C) in patients with unknown EGFR mutation status. PFS, progression-free survival.

Fig. 4
Overall survival curves according to pretreatment serum levels of carcinoembryonic antigen (CEA) and cytokeratin-19 fragments (CYFRA 21-1). (A) CEA. (B) CYFRA 21-1. (C) Combinations of CEA and CYFRA 21-1 by group: a, patients with a low level of CEA and a high level of CYFRA 21-1; b, patients with both low or high level of CEA and CYFRA 21-1; and c, patients with a high level of CEA and a low level of CYFRA 21-1. OS, overall survival.

Table 1
Comparison of Pretreatment Clinicopathological Characteristics according to CEA and CYFRA 21-1 Levels

Table 2
Comparison of Pretreatment Clinicopathological Characteristics according to EGFR TKI Responses
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine for 6-2007-0194.
References
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008. 58:71–96.

2. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005. 353:123–132.

3. Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008. 372:1809–1818.

4. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009. 361:947–957.

5. Maheswaran S, Sequist LV, Nagrath S, Ulkus L, Brannigan B, Collura CV, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008. 359:366–377.

6. Molina R, Auge JM, Escudero JM, Marrades R, Viñolas N, Carcereny E, et al. Mucins CA 125, CA 19.9, CA 15.3 and TAG-72.3 as tumor markers in patients with lung cancer: comparison with CYFRA 21-1, CEA, SCC and NSE. Tumour Biol. 2008. 29:371–380.

7. Okada M, Nishio W, Sakamoto T, Uchino K, Yuki T, Nakagawa A, et al. Prognostic significance of perioperative serum carcinoembryonic antigen in non-small cell lung cancer: analysis of 1,000 consecutive resections for clinical stage I disease. Ann Thorac Surg. 2004. 78:216–221.

8. Pujol JL, Boher JM, Grenier J, Quantin X. Cyfra 21-1, neuron specific enolase and prognosis of non-small cell lung cancer: prospective study in 621 patients. Lung Cancer. 2001. 31:221–231.

9. Holdenrieder S, von Pawel J, Dankelmann E, Duell T, Faderl B, Markus A, et al. Nucleosomes and CYFRA 21-1 indicate tumor response after one cycle of chemotherapy in recurrent non-small cell lung cancer. Lung Cancer. 2009. 63:128–135.

10. Ardizzoni A, Cafferata MA, Tiseo M, Filiberti R, Marroni P, Grossi F, et al. Decline in serum carcinoembryonic antigen and cytokeratin 19 fragment during chemotherapy predicts objective response and survival in patients with advanced nonsmall cell lung cancer. Cancer. 2006. 107:2842–2849.

11. Okamoto T, Nakamura T, Ikeda J, Maruyama R, Shoji F, Miyake T, et al. Serum carcinoembryonic antigen as a predictive marker for sensitivity to gefitinib in advanced non-small cell lung cancer. Eur J Cancer. 2005. 41:1286–1290.

12. Barlési F, Tchouhadjian C, Doddoli C, Torre JP, Astoul P, Kleisbauer JP. CYFRA 21-1 level predicts survival in non-small-cell lung cancer patients receiving gefitinib as third-line therapy. Br J Cancer. 2005. 92:13–14.

13. Chiu CH, Shih YN, Tsai CM, Liou JL, Chen YM, Perng RP. Serum tumor markers as predictors for survival in advanced non-small cell lung cancer patients treated with gefitinib. Lung Cancer. 2007. 57:213–221.

14. Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E. Histological typing of lung and pleural tumours. 1999. 3rd ed. Berlin: Springer-Verlag.
15. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.
16. Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005. 23:2493–2501.

17. Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965. 121:439–462.

18. Fujishima T, Honda Y, Shijubo N, Takahashi H, Abe S. Increased carcinoembryonic antigen concentrations in sera and bronchoalveolar lavage fluids of patients with pulmonary alveolar proteinosis. Respiration. 1995. 62:317–321.

19. Rule AH, Straus E, Vandevoorde J, Janowitz HD. Tumor-associated (CEA-reacting) antigen in patients with inflammatory bowel disease. N Engl J Med. 1972. 287:24–26.

20. Okada M, Nishio W, Sakamoto T, Uchino K, Yuki T, Nakagawa A, et al. Effect of histologic type and smoking status on interpretation of serum carcinoembryonic antigen value in non-small cell lung carcinoma. Ann Thorac Surg. 2004. 78:1004–1009.

21. Matsuoka K, Sumitomo S, Nakashima N, Nakajima D, Misaki N. Prognostic value of carcinoembryonic antigen and CYFRA21-1 in patients with pathological stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2007. 32:435–439.

22. Shoji F, Yoshino I, Yano T, Kometani T, Ohba T, Kouso H, et al. Serum carcinoembryonic antigen level is associated with epidermal growth factor receptor mutations in recurrent lung adenocarcinomas. Cancer. 2007. 110:2793–2798.

23. Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989. 57:327–334.

24. Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999. 9:67–81.

25. Screaton RA, Penn LZ, Stanners CP. Carcinoembryonic antigen, a human tumor marker, cooperates with Myc and Bcl-2 in cellular transformation. J Cell Biol. 1997. 137:939–952.

26. Ordoñez C, Screaton RA, Ilantzis C, Stanners CP. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res. 2000. 60:3419–3424.
27. Stieber P, Bodenmüller H, Banauch D, Hasholzner U, Dessauer A, Ofenloch-Hähnle B, et al. Cytokeratin 19 fragments: a new marker for non-small-cell lung cancer. Clin Biochem. 1993. 26:301–304.

28. Nisman B, Lafair J, Heching N, Lyass O, Baras M, Peretz T, et al. Evaluation of tissue polypeptide specific antigen, CYFRA 21-1, and carcinoembryonic antigen in nonsmall cell lung carcinoma: does the combined use of cytokeratin markers give any additional information? Cancer. 1998. 82:1850–1859.

29. Barlési F, Gimenez C, Torre JP, Doddoli C, Mancini J, Greillier L, et al. Prognostic value of combination of Cyfra 21-1, CEA and NSE in patients with advanced non-small cell lung cancer. Respir Med. 2004. 98:357–362.

30. Pujol JL, Molinier O, Ebert W, Daurès JP, Barlesi F, Buccheri G, et al. CYFRA 21-1 is a prognostic determinant in non-small-cell lung cancer: results of a meta-analysis in 2063 patients. Br J Cancer. 2004. 90:2097–2105.

31. Kao CH, Hsieh JF, Ho YJ, Tsai SC, Lee JK. Cytokeratin fragment 19 (CYFRA 21-1) in healthy smokers. Anticancer Res. 1999. 19:4545–4546.
32. Molina R, Filella X, Augé JM, Fuentes R, Bover I, Rifa J, et al. Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis. Comparison with the main clinical and pathological prognostic factors. Tumour Biol. 2003. 24:209–218.

33. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005. 97:339–346.

34. Gaughan EM, Costa DB. Genotype-driven therapies for non-small cell lung cancer: focus on EGFR, KRAS and ALK gene abnormalities. Ther Adv Med Oncol. 2011. 3:113–125.