Abstract
Purpose
We evaluated whether the clinicopathological factors of papillary thyroid microcarcinoma (PTMC), especially tumoe size, are associated with subcinical central lymph node metastasis.
Materials and Methods
A total of 160 patients diagnosed with PTMC who underwent total thyroidectomy with bilateral central lymph node dissection were enrolled in this study. All patients were clinically lymph node negative PTMC. Patients were divided into 2 groups according to the size of tumor (≤5 mm vs. >5 mm). Clinicopathologic risk factors for subclinical central lymph node metastasis were analyzed.
Results
Subclinical central lymph node metastasis was detected in 61 (38.1%). Patients with tumors ≤5 mm had a lower frequency of extrathyroidal extension, multifocality and subclinical central lymph node metastasis. On multivariate analysis, only male and tumor size >5 mm were independent predictors of subclinical central lymph node metastasis; age, multifocality, bilaterality, extrathyroidal extension, lymphvascular invasion and lymphocytic thyroiditis were not.
Conclusion
In this study, male and tumor size >5 mm were two independent predictive factors for subclinical central lymph node metastasis in PTMC. These are easier factors to assess before surgery than other factors when planning the central lymph node dissection. However, further long-term follow-up studies are needed to confirm the prognostic significance of subclinical central lymph node metastasis in PTMC.
In 2008, according to the National Cancer Registry data of Korea, thyroid cancer was reported as the most prevalent cancer in women.1 In particular, papillary thyroid microcarcinoma (PTMC) is rapidly increasing.
Although PTMC generally has a highly favorable prognosis, the long term recurrence rate of PTMC has been reported as up to 10%.2 The majority of PTMC recurrence are locoregional, in the thyroid bed and in the neck lymph node.3 Central lymph node metastasis is an important risk factor of recurrence, and often is not detected clinically. Prevalence of the subclinical central lymph node metastasis has been reported to be as great as 30-65% in PTMC; however, the role of routine central lymph node dissection in the treatment of PTMC remains debated.4,5 Routine prophylactic central lymph node dissection may be overtreatment in many patients with PTMC. Therefore, identification of predictive factors associated with subclinical central lymph node metastasis may help tailor appropriate surgical strategies for patients with PTMC.
In addition, endoscopic thyroidectomy is rapidly increasing in Korea for preoperatively diagnosed low-risk PTMC due to the attractive advantage of good cosmetic results in Korea.6,7 However, endoscopic thyroidectomy has some limitations such as a difficulty in achieving sufficient lymphadenectomy and a narrower operative space.6,7 Therefore, knowing about the absence or presence of subclinical central lymph node metastasis can help physicans choose an appropriate method and extent of surgery.
Recently, tumors ≤5 mm along their great dimension in PTMC are increasing as a result of earlier diagnosis by high resolution ultrasonographic examination and skillful fine needle aspiration biopsy as part of the routine health check-ups.8 Results of studies on whether the tumor size is an independent predictive factor for the presence of subclinical central lymph node metastasis or not are not consistent.9,10
We thought, therefore, that if there was any difference found in subclinical central lymph node metastasis on the basis of clinicopathologic features, especially the tumor size of PTMC, we could administer more aggressive treatment to the group of patients who have more aggressive PTMC. Therefore, the aim of our study was to find out which factors are associated with subclinical central lymph node metastasis of PTMC, and also to test whether the diameter of the carcinoma is a significant prognostic factor for the lymph node metastasis.
A total of 160 patients diagnosed with PTMC who underwent total thyroidectomy with bilateral central lymph node metastasis between January 2006 and July 2010 were enrolled in this study. Of the 160 patients, 31 (19.4%) patients underwent endoscopic surgery. Based on findings from preoperative ultrasonography and fine needle aspiration biopsy, all patients were clinically lymph node negative PTMC. Bilteral central lymph node dissection was defined as bilateral dissection of bilateral paratracheal, pretracheal and prelaryngeal lymph nodes. Diagnosis of PTMC was reconfirmed by the surgical pathology for all patients. PTMC was defined as tumor 10 mm or less along its greatest dimension, in accordance with the histologic classification of thyroid tumors by the World Health Organization.11 This study was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital.
We carried out retrospective chart review of all patients treated at our institution for total thyroidectomy with central lymph node dissection. Various clinicopathologic prognostic factors described in literature such as age, gender, tumor size, extrathyroidal extension, multifocality, bilaterality and central lymph node dissection were reviewed. Lymphovascular invasion and lymphocytic thyroiditis were also recorded. Patients were classified into two groups; those with PTMC tumor size ≤5 mm and those with PTMC >5 mm as done in previous studies. The extent of bilateral central neck dissection was similar between those 2 groups. In cases with the presence of more than two malignancies, multifocality was defined as multiple malignancies in one lobe and bilaterality was defined as multiple malignancies in both lobes of thyroid.
Data are presented as the mean±SD. SPSS 14.0 for Windows® software package (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Student t-test was used to compare the clinicopathologic factors between the tumor size ≤5 mm group and >5 mm group.
Univariate analysis was used for the assessment of the factors associated with subclinical central lymph node dissection. Statistically significant results obtained at univariate analysis were submitted to multivariate logistic regression. A p-value of <0.05 was considered statistically significant.
Of 160 patients, 19 (9.1%) were men and 141 (91.9%) were women. The mean age at initial treatment was 47 years (range, 20 to 78 years). The largest dimensions of primary tumors ranged from 2 to 10 mm, with a mean of 6.8 mm. Subclinical central lymph node dissection was detected in 61 (38.1%) of 160 patients, with clinically node-negative PTMC. Multifocal lesions were found in 54 (33.8%) and bilateral lesions in 37 (23.1%) patients. Extrathyroidal and lymphovascular invasion of primary tumors were detected in 82 (51.3%), and 4 (2.5%) patients, respectively. Lymphocytic thyroiditis was detected in 66 (40.7%). Proportion of endoscopic thyroidectomy was 19.4% (31 to 160) (Table 1). In 61 patients with subclinical central lymph node metastasis, the mean number of metastatic lymph nodes was 2.6±2.9 (data not shown).
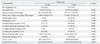
The size of the thyroid cancer was 5 mm or less in 41 patients (25.6%), and larger than 5 mm in 119 patients (74.4%). Table 2 compares the differences in clinicopathologic features between patients with PTMC ≤5 mm and the patients with PTMC >5 mm. Patients with tumors ≤5 mm had a lower frequency of extrathyroidal extension than the patients with tumors >5 mm (31.7% vs. 58.0%, p=0.003). Also, patients with tumors ≤5 mm had a lower frequency of multifocality than patients with tumors >5 mm (22% vs. 37.8%, p=0.046). All patients with lymphovascular invasion had tumors >5 mm. However, two groups did not have significant differences in age, bilaterality, number of removed lymph node, lymphocytic thyroiditis and proportion of endoscopic thyroidectomy.
The frequency of central lymph node metastasis was greater in male patients (p=0.005), and in patients with tumor size >5 mm (p<0.001). The presence of extrathyroidal extension and lymphovascular invasion were statistically related to subclinical central lymph node metastasis (p=0.021 and p=0.02, respectively). However, age, bilaterality, multifocality of tumor and lymphocytic thyroiditis were not significantly related to subclinical central lymph node metastasis (Table 3). Logistic regression analysis identified the most influential variables associated with subclinical central lymph node metastasis. On multivariate analysis, only male gender (odds ratio, 6.022; 95% CI 1.86-19.55) and tumor size >5 mm (odds ratio, 4.550; 95% CI 1.67-12.38) were independent predictors of subclinical central lymph node metastasis (Table 4).
Results from this study showed that male gender and tumor size >5 mm were independent predictors of subclinical central lymph node metastasis. Age, multifocality, bilaterality, extrathyroidal extension, lymphovascular invasion and lymphocytic thyroiditis were not predictive of subclinical central lymph node metastasis.
Despite good overall prognosis of PTMC, recurrence of the disease after initial surgical cure remains a troublesome problem.9,12,13 According to the study by Hay, et al.,14 the recurrence within cervical lymph nodes was more than 80%. They noted "nodes beget nodes". Central lymph node metastasis is believed to be related to poor prognosis because they occurs spontaneously or precede the occurrence of distant metastases.15 Despite the absence of palpable neck nodes, PTMC has a considerable rate of lymph node metastasis to the central compartment.3-5 Our study showed that subclinical central lymph node metastasis was detected in 38.1% of cases. Previous studies showed results similar to those of our study.5,16 In contrast, the studies of Pakdaman, et al.17 and Lee, et al.18 showed lower frequencies than our study.
The indications of central lymph node dissection are another major issue in PTMC management and debate goes on regarding the optimal treatment for this disease. Routine prophylactic central lymph node dissection for PTMC has been in debated because prophylactic central lymph node dissection seems to have little prognostic benefit.3,4 However, central lymph node metastasis is an important risk factor of recurrence, and often is not detected clinically. Thus, it is reasonable to perform elective central lymph node dissection after predicting the presence of subclinical central lymph node metastasis. If we can predict central lymph node metastasis through assessing clinical factors, it would be a useful information to help us decide on the extent of surgery.
Several studies have described clinicopathological factors associated with subclinical central lymph node metastasis in patients with PTMC, but results from those studies were inconsistent. In particular, it is considered important whether or not tumor size is predictor of subclinical central lymph node metastasis. Although assessment of tumor size depends on a large number of uncontrolled variables, high resolution ultrasonography by skilled physicians give good images and can be used to assess the size of tumor. Because we can know approximate tumor size by high resolution ultrasonography and can plan the extent of surgery ahead at preoperation. The present study showed that subclinical central lymph node metastasis is significantly associated with tumor size in univariate analysis and tumor size is an independent predictor of subclinical central lymph node metastasis in multivariate analysis. Kasai and Sakamoto19 reported that patients with a tumor size >5 mm had central lymph node metastasis more frequently than with the patients with a tumor size ≤5 mm. Also, several authors have demonstrated that PTMC tumors >5 mm are more significantly associated with central lymph node metastasis than with those of less than 5 mm.4,18,20 In addition, our study showed that a PTMC >5 mm related to signs of tumor aggressiveness (multifocality, extrathyroidal extension) in agreement with other studies. In contrast to our study, however, studies by So, et al.16 and Roh, et al.5 reported that tumor size was not an independent predictor of subclinical central lymph node metastasis in multivariate analysis.
Recently, several authors subdivided tumor size using various cutoff values. Recently, Lee, et al.21 divided the patients into two groups using cutoff values of 5-9 mm; ones with tumors less than the cutoff value and the other with tumors at or more than the cutoff value. They reported that PTMC aggressiveness is present mainly in patients with PTMC >7 mm and a tumor size >7 mm was an independent factor associated with central lymph node metastasis. In the study by Pakdaman, et al.,17 a threshold of ≥4 mm was found to be more significant than one of 5 mm for conferring increased risk for extrathyroidal spread. However, they did not study differences in lymph node metastasis according to cutoff of sizes of 5 mm vs. 4 mm. Up to now, many studies have analyzed the aggressiveness of PTMC based on a size of 5 mm. The debate as to the clinical implications of PTMC especially <5 mm in size, and the necessity for central lymph node dissection will continue.
We found no relation between tumor multifocaltiy, bilaterality and central lymph node metastasis.4,16,22 In the study by Hay, et al.14 multifocality increased the risk of later nodal recurrence, with 11% of multifocal tumors exhibiting recurrence, compared with only 4% of unifocal tumors. On the other hand, Roh, et al.,5 Lee, et al.18 and Lee, et al.23 reported that there was no relation between tumor multifocality and central lymph node metastasis.
In the present study, there was no association between age and subclinical central lymph node metastasis, in consistent with some earlier studies.5,16,24
Our finding of male gender as an unfavorable prognostic factor coincides with previous studies identifying the role of gender as an important demographic variable in risk stratification for patients with papillary thyroid carcinoma (PTC).18,25,26 Previous studies found inconsistent results regarding the association between gender and lymph node metastasis, and Roh, et al.5 reported that there was no association between male gender and central lymph node metastasis.
Extrathyroidal extension of thyroid carcinoma was associated with poor prognosis. Extrathyroidal extension was found in 51.3% of patients in our study, and these results are very similar to other studies.16 Most cases of extrathyroidal extension in our study showed minimal invasion of perithyroidal soft tissue or strap muscle. Not only gross invasion into extrathyroidal structures but also microscopic extension outside of thyroid capsule is included in the diagnosis of extrathyroidal extension in this study as well as in many other Korean studies.16 Although extrathyroidal extension is well known as a prognostic factor in differentiated thyroid carcinoma, minimal extrathyroidal extension has not been associated with recurrence in a few studies.27 In this study, extrathyroidal extension was statistically significantly associated with tumor size and subclinical central lymph node metastasis, but extrathyroidal extension was not an independent predictive factor for subclinical central lymph node metastasis in multivariate analysis. Moon, et al.28 showed that minimal extrathyroidal extension was significantly associated with tumor size and central lymph node metastasis.
It is known that the prevalence of lymphocytic thyroiditis is higher in patients with PTC.29 The effect of coexistant lymphocytic thyroiditis on prognosis in PTC patients remains controversial and only a few studies have been performed in PTMC patients.30 Kim, et al.31 noted that 15% of patients with PTC of mean tumor size 2.2 cm had lymphocytic thyroiditis, and lymphocytic thyroiditis was associated with smaller size of the primary tumor at presentation. However, there were no differences in the prevalence of central lymph node metastasis according to the presence of lymphocytic thyroiditis. In a PTMC study, So, et al.16 reported that 24.9% of 551 patients with PTMC had lymphocytic thyroiditis and there was no association between lymphocytic thyroiditis and lymph node metastasis, and Kim, et al.32 showed that the presence of lymphocytic thyroiditis did not influence the frequency of lymph node metastasis in all PTMC cases. In our study, the prevalence of lymphocytic thyroiditis was higher than in other studies, and there was no association between lymphocytic thyroiditis and central lymph node metastasis.
Lymphovascular invasion was not associated with central lymph node metastasis in our study. Kim, et al.33 reported that lymphovascular invasion was associated not with central lymph node metastasis but with lateral cervical lymph node metastasis in patients with PTC. Our results are consistent with other studies for patients with PTMC.5,16,33
Our study has several limitations that must be taken into account. First, since this study was a retrospective analysis, the prognostic significance of subclinical lymph node metastasis such as the relation to tumor recurrence has not been fully investigated. The long-term follow-up studies are needed to confirm the prognostic significance of subclinical central lymph node metastasis in PTMC. Second, our study population was a cohort of patients cared for in a single center. Therefore, it is unlikely that this cohort of small provides reliable statements, and a much larger number of subjects in multicenter will be needed to generalize this results. In conclusion, in the present study, male gender and tumor size greater than 5 mm were two independent predictive factors for subclinical central lymph node metastasis in PTMC. These are factors to more easily assess before surgery than other factors when planning the central lymph node dissection. However, these data should be interpreted with caution because of retrospective analysis and lack of data for the prognostic significance.
Figures and Tables
Table 2
Clinical Characteristics at Presentation, Classified Into Two Subgroups According to the Tumor Size
References
1. Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011. 43:1–11.

2. Giordano D, Gradoni P, Oretti G, Molina E, Ferri T. Treatment and prognostic factors of papillary thyroid microcarcinoma. Clin Otolaryngol. 2010. 35:118–124.

3. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg. 2006. 30:91–99.

4. Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003. 237:399–407.
5. Roh JL, Kim JM, Park CI. Lateral cervical lymph node metastases from papillary thyroid carcinoma: pattern of nodal metastases and optimal strategy for neck dissection. Ann Surg Oncol. 2008. 15:1177–1182.

6. Koh YW, Park JH, Kim JW, Lee SW, Choi EC. Endoscopic hemithyroidectomy with prophylactic ipsilateral central neck dissection via an unilateral axillo-breast approach without gas insufflation for unilateral micropapillary thyroid carcinoma: preliminary report. Surg Endosc. 2010. 24:188–197.

7. Chung YS, Choe JH, Kang KH, Kim SW, Chung KW, Park KS, et al. Endoscopic thyroidectomy for thyroid malignancies: comparison with conventional open thyroidectomy. World J Surg. 2007. 31:2302–2306.

8. Pelizzo MR, Boschin IM, Toniato A, Pagetta C, Piotto A, Bernante P, et al. Natural history, diagnosis, treatment and outcome of papillary thyroid microcarcinoma (PTMC): a mono-institutional 12-year experience. Nucl Med Commun. 2004. 25:547–552.

9. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994. 97:418–428.

10. Hay ID, Grant CS, Taylor WF, McConahey WM. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987. 102:1088–1095.
11. Hedinger C, Williams ED, Sobin LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. 1989. 63:908–911.

12. Appetecchia M, Scarcello G, Pucci E, Procaccini A. Outcome after treatment of papillary thyroid microcarcinoma. J Exp Clin Cancer Res. 2002. 21:159–164.
13. Hay ID, Grant CS, van Heerden JA, Goellner JR, Ebersold JR, Bergstralh EJ. Papillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year period. Surgery. 1992. 112:1139–1146.
14. Hay ID, Hutchinson ME, Gonzalez-Losada T, McIver B, Reinalda ME, Grant CS, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008. 144:980–987.

15. Harwood J, Clark OH, Dunphy JE. Significance of lymph node metastasis in differentiated thyroid cancer. Am J Surg. 1978. 136:107–112.

16. So YK, Son YI, Hong SD, Seo MY, Baek CH, Jeong HS, et al. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery. 2010. 148:526–531.

17. Pakdaman MN, Rochon L, Gologan O, Tamilia M, Garfield N, Hier MP, et al. Incidence and histopathological behavior of papillary microcarcinomas: study of 429 cases. Otolaryngol Head Neck Surg. 2008. 139:718–722.

18. Lee NS, Bae JS, Jeong SR, Jung CK, Lim DJ, Park WC, et al. Risk factors of lymph node metastasis in papillary thyroid microcarcinoma. J Korean Surg Soc. 2010. 78:82–86.

20. Roti E, Rossi R, Trasforini G, Bertelli F, Ambrosio MR, Busutti L, et al. Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab. 2006. 91:2171–2178.

21. Lee KJ, Cho YJ, Kim SJ, Lee SC, Kim JG, Ahn CJ, et al. Analysis of the clinicopathologic features of papillary thyroid microcarcinoma based on 7-mm tumor size. World J Surg. 2011. 35:318–323.

22. Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer. 2003. 98:31–40.

23. Lee SH, Lee SS, Jin SM, Kim JH, Rho YS. Predictive factors for central compartment lymph node metastasis in thyroid papillary microcarcinoma. Laryngoscope. 2008. 118:659–662.

24. Lim YC, Choi EC, Yoon YH, Kim EH, Koo BS. Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br J Surg. 2009. 96:253–257.

25. Shaha AR, Shah JP, Loree TR. Risk group stratification and prognostic factors in papillary carcinoma of thyroid. Ann Surg Oncol. 1996. 3:534–538.

26. Besic N, Pilko G, Petric R, Hocevar M, Zgajnar J. Papillary thyroid microcarcinoma: prognostic factors and treatment. J Surg Oncol. 2008. 97:221–225.

27. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Minimal extrathyroid extension does not affect the relapse-free survival of patients with papillary thyroid carcinoma measuring 4 cm or less over the age of 45 years. Surg Today. 2006. 36:12–18.

28. Moon HJ, Kim EK, Chung WY, Yoon JH, Kwak JY. Minimal extrathyroidal extension in patients with papillary thyroid microcarcinoma: is it a real prognostic factor? Ann Surg Oncol. 2011. 18:1916–1923.

29. Matsubayashi S, Kawai K, Matsumoto Y, Mukuta T, Morita T, Hirai K, et al. The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab. 1995. 80:3421–3424.

30. Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 1999. 84:458–463.

31. Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, et al. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2009. 71:581–586.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download