Abstract
Eperisone and afloqualone act by relaxing both skeletal and vascular smooth muscles to improve circulation and suppress pain reflex. These drugs are typically prescribed with non-steroidal anti-inflammatory drugs (NSAIDs) as painkillers. However, there have been no reports on serious adverse reactions to oral muscle relaxants; and this is the first report to describe three allergic reactions caused by eperisone and afloqualone. All three patients had histories of allergic reactions after oral intake of multiple painkillers, including oral muscle relaxants and NSAIDs, for chronic muscle pain. An open-label oral challenge test was performed with each drug to confirm which drugs caused the systemic reactions. All patients experienced the same reactions within one hour after oral intake of eperisone or afloqualone. The severity of these reactions ranged from laryngeal edema to hypotension. To confirm that the systemic reaction was caused by eperisone or afloqualone, skin prick testing and intradermal skin tests were performed with eperisone or afloqualone extract in vivo, and basophil activity tests were performed after stimulation with these drugs in vitro. In one patient with laryngeal edema, the intradermal test with afloqualone extract had a positive result, and CD63 expression levels on basophils increased in a dose-dependent manner by stimulation with afloqualone. We report three allergic reactions caused by oral muscle relaxants that might be mediated by non-immunoglobulin E-mediated responses. Since oral muscle relaxants such as eperisone and afloqualone are commonly prescribed for chronic muscle pain and can induce severe allergic reactions, we should prescribe them carefully.
In Korea, eperisone and afloqualone together with non-steroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed to control muscle pain. This is the first report of three anaphylactic reactions caused by eperisone and afloqualone.
A 54-year-old woman with shoulder pain experienced two anaphylactic episodes after oral intake of several painkillers, including eperisone and diclofenac. She had no history of other allergic diseases, but had taken an angiotensin receptor antagonist and metformin to control hypertension and diabetes for several years. Her anaphylactic reactions included generalized urticaria, angioedema, dyspnea, hypotension, and dizziness. She visited the emergency room in both episodes and her symptoms were relieved after proper management.
She had no peripheral eosinophilia, although serum total immunoglobulin E (IgE) was elevated to 922 IU/mL. Skin prick testing (SPT) for common inhalant allergens showed all negative responses. Open-label oral challenge tests were performed with eperisone and diclofenac separately to identify the causative drug. After taking 50 mg of eperisone, a systemic reaction developed within 30 min, which included generalized urticaria, cyanosis, and dizziness. The patient's blood pressure dropped from 110/70 mmHg to 60/30 mmHg. She recovered fully after immediate administration of epinephrine, corticosteroid, and antihistamines. The results of SPT and an intradermal skin test with eperisone extract were negative. Since another challenge test with diclofenac also gave a negative result, she was confirmed as having an eperisone-induced anaphylactic reaction.
A 62-year-old woman visited the emergency room with dyspnea and angioedema after taking painkillers, including eperisone and meloxicam, to treat back pain. She had been taking atenolol and enalapril for hypertension for over 15 years, and had no history of any allergic reactions or other medical disease.
The patient's chest X-ray, electrocardiogram (ECG), and routine laboratory findings were normal. She had no peripheral eosinophilia, although serum total IgE levels were elevated to 401 IU/ML, and serum tryptase level was elevated to 32.5 µg/L. After recovery, open-label oral challenge tests were performed with eperisone and meloxicam separately. After taking 50 mg of eperisone, generalized urticaria and itching developed within one hour, and her blood pressure decreased from 150/100 mmHg to 120/80 mmHg.
SPT and an intradermal skin test with eperisone extract were performed, and results were all negative. An additional oral challenge test with meloxicam gave a negative result. Therefore, she was confirmed as having an eperisone-induced anaphylactic reaction.
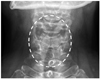
A 56-year-old woman experienced angioedema, hoarseness, and dyspnea twice within 10 min after ingesting multiple painkillers, including afloqualone and talniflumate. She had no history of other allergic diseases and long-term medications. The ECG, radiological, and routine laboratory findings were normal. She had no peripheral eosinophilia, and serum total IgE levels were within normal limits. SPT to common inhalant allergens gave all negative responses. Open-label oral challenge tests were performed with afloqualone and talniflumate separately. After taking 20 mg of afloqualone, a systemic reaction, including angioedema, nasal obstruction, hoarseness, and breathing difficulty, developed within 10 min. The patient's vital signs were stable, but stridor was heard in the anterior chest. A neck X-ray showed laryngeal edema (Fig. 1). Her symptoms improved after treatment with corticosteroid and antihistamines. Although SPT for afloqualone was negative, the intradermal test gave a positive response to 1 mg/mL afloqualone with a mean wheal size of 5 mm. The basophil activity test was performed to verify that the drug caused the systemic reaction. The maximum CD63 levels from basophils obtained from the patient occurred upon incubation with 0.25 mg/mL of afloqualone (46.1%) compared with the baseline value (25.5%) (Fig. 2). An oral challenge test with talniflumate gave a negative result.
The patient was confirmed as having an allergic reaction to afloqualone, based on positive results in an open-label oral challenge test, intradermal test, and basophil activity test.
Eperisone and afloqualone are commonly prescribed oral muscle relaxants.1,2 They act centrally by relaxing skeletal and vascular smooth muscles to improve circulation and suppress pain reflex.3,4 These drugs are usually prescribed in combination with NSAIDs as painkillers. Although there have been a few reports of drug eruptions caused by eperisone, such as a fixed drug eruption,5 erythema, and angioedema,6 there has been no report of systemic adverse events, including anaphylactic reactions, to eperisone. There has been a single report of anaphylactic reactions caused by tolperisone, which is closely related to eperisone.7 In addition, there have been a few reports on afloqualone-induced drug eruptions limited to the skin.8 Therefore, this is the first report describing serious systemic adverse reactions, including anaphylaxis and laryngeal edema, caused by eperisone and afloqualone.
The first two cases were anaphylactic reactions that occurred within 30 min after taking 50 mg of eperisone, and they were confirmed by oral provocation tests. In the second case, this anaphylactic reaction could be augmented by daily intake of a beta-blocker and angiotensin-converting enzyme inhibitor, as verified by increased serum tryptase level. In the third case, laryngeal edema developed after taking afloqualone, which was confirmed by a neck radiograph, and the reaction was verified by positive responses to the intradermal test and basophil activity test, induced by afloqualone extract. Although there are no reports describing the underlying mechanisms of these allergic reactions, our findings suggest that the oral muscle relaxants described herein could directly activate basophils to induce severe systemic reactions.
Oral muscle relaxants are commonly prescribed with NSAIDs to relieve pain. Therefore, practitioners might assume that the causative drug is an NSAID, since aspirin and NSAIDs are the most common cause of anaphylaxis.9 However, since newly developed oral muscle relaxants, such as eperisone and afloqualone, can induce systemic allergic reactions, all practitioners should bear in mind that oral muscle relaxants could cause drug-induced anaphylactic reactions.
Figures and Tables
References
1. Matsunaga M, Uemura Y, Yonemoto Y, Kanai K, Etoh H, Tanaka S, et al. Long-lasting muscle relaxant activity of eperisone hydrochloride after percutaneous administration in rats. Jpn J Pharmacol. 1997. 73:215–220.

2. Ochiai T, Ishida R. Pharmacological studies on 6-amino-2-fluoromethyl-3-(O-tolyl)-4(3H)-quinazolinone (afloqualone), a new centrally acting muscle relaxant. (II) Effects on the spinal reflex potential and the rigidity. Jpn J Pharmacol. 1982. 32:427–438.

3. Inoue S, Bian K, Okamura T, Okunishi H, Toda N. Mechanisms of action of eperisone on isolated dog saphenous arteries and veins. Jpn J Pharmacol. 1989. 50:271–282.

4. Iwase S, Mano T, Saito M, Ishida G. Effect of a centrally-acting muscle relaxant, eperisone hydrochloride, on muscle sympathetic nerve activity in humans. Funct Neurol. 1992. 7:459–470.
5. Choonhakarn C. Non-pigmenting fixed drug eruption: a new case due to eperisone hydrochloride. Br J Dermatol. 2001. 144:1288–1289.

6. Ueno T, Kawana S. [A case of eperisone hydrochloride (myonal)--induced drug eruption leading to erythema and angioedema]. Arerugi. 2007. 56:709–713.
7. Ribi C, Vermeulen C, Hauser C. Anaphylactic reactions to tolperisone (Mydocalm). Swiss Med Wkly. 2003. 133:369–371.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download