Abstract
Acute disseminated encephalomyelitis (ADEM) is a monophasic autoimmune demyelinating disease of the central nervous system, which typically follows acute viral or bacterial infection or vaccination. We report a case of ADEM associated with hepatitis C virus (HCV) infection with positive serum and cerebrospinal fluid (CSF) anti-HCV antibody. After steroid treatment, neurologic symptoms were improved. Virus triggers autoimmunity or direct viral invasion plays a part in the genesis of ADEM. This is the first reported case of ADEM with anti-HCV antibody in the CSF.
Acute disseminated encephalomyelitis (ADEM) is an immune-mediated inflammatory disorder of the central nervous system, which typically follows acute viral or bacterial infection or vaccination. ADEM is characterized by widespread demyelination that predominantly involves the white matter of the brain and spinal cord. Numerous infectious agents, mostly nonspecific upper respiratory tract infections, have been linked to ADEM. Hepatitis virus is a rare cause of ADEM. One case of hepatitis C virus (HCV) and two cases of hepatitis A virus with ADEM have been reported.1-3
We report a case of ADEM associated with HCV infection. This is the first case of ADEM with anti-HCV antibody in the cerebrospinal fluid (CSF).
A 47-year-old woman was admitted to our hospital for dysarthria and left-sided hypesthesia. She had no history of recent febrile illness or vaccination. Two weeks after the first symptoms, she developed right-sided weakness. On admission, she had no fever and normal blood pressure. Electrocardiography and chest X-ray were normal. Laboratory tests showed that anti-HCV antibody was positive and HCV RNA titer was highly elevated (1,660,000 IU/mL). AST and ALT were also mildly elevated (34/44 IU/L). Her medical history revealed that she had blood transfusion during Cesarean section about twenty years ago. She had no symptoms of hepatitis. Serologic tests, including viral markers, showed nothing significant. Levels of anti-cardiolipin antibody, anti-nuclear antibody, and anti-neutrophil cytoplasmic antibody were also normal. Cancer antigen 19-9, was mildly elevated (27.7 U/mL; normal range, 0.8-24.0 U/mL). The CSF examination showed three white blood cells with mildly increased protein (53 mg/dL; normal range, 15-45 mg/dL), normal IgG index, and no IgG oligoclonal bands. CSF anti-HCV antibody was positive. Magnetic resonance imaging (MRI) of the brain and spine revealed multifocal high signal intensity lesions on fluid attenuated inversion recovery and T2-weighted images in the brainstem, thalamus, and cervical and thoracic spinal cord (Fig. 1). Some lesions showed enhancement after gadolinium administration.
Treatment with intravenous methylprednisolone, 1 g daily for 5 days, was instituted. The intravenous methylprednisolone was changed to oral prednisone, 60 mg for 1 week, and then reduced to 10 mg per week. Two weeks after steroid treatment, neurologic symptoms and spine MRI improved further. However, brain MRI had no significant interval change. Follow-up HCV RNA titer increased about three-folds (4,900,000 IU/mL). Liver biopsy demonstrated stage 2 of fibrosis. It was reasonable for chronic C viral hepatitis. At discharge two months after admission, neurologic symptoms and spine and brain MRI (Fig. 2) improved. Her neurologic examination was nearly normal except left side limb ataxia.
HCV infection is a common and chronic disorder with numerous extrahepatic manifestations.4 It can involve the renal, dermatologic, hematologic, rheumatologic, or nervous system, however, rarely involves the central nervous system (CNS). CNS involvement has been reported with HCV-associated vasculitis,5-7 progressive encephalomyelitis with rigidity,8 and transverse myelitis.9,10 ADEM associated with HCV has been reported before, but anti-HCV antibody was not detected in CSF in that case.1
While the pathogenetic mechanism of ADEM is still obscure, a virus invasion or virus induced immune process can be suggested. Primary autoimmune responses and immune response secondary to an infection may contribute to CNS inflammation with subsequent demyelination. A number of infectious agents, mainly viruses, have been shown to be associated with ADEM, including coronavirus, coxsackie virus B, Epstein-Barr virus, herpes simplex virus, human herpes virus 6, measles, mumps, rubella, borrelia burgdorferi, chlamydia, legionella, and mycoplasma pneumoniae.11
More than half of the HCV-infected patients may be related to autoimmunity associated with HCV. In our patient, HCV RNA titer was highly increased, and it was compatible with chronic C viral hepatitis on liver biopsy. During Cesarean section twenty years earlier, she had had multiple blood transfusions. Recently, however, she did not receive blood transfusion or any injection. Therefore, we suppose that the chronic HCV infection was the cause of ADEM, and that virus-triggered autoimmunity or direct viral invasion played a role in the genesis of ADEM. In a case of acute transverse myelitis associated with chronic HCV infection, anti-HCV antibody was present in the CSF.10 In our case, anti-HCV antibody was also detected in the CSF. It is hypothesized that the virus might have penetrated blood brain barrier and involved the CNS directly.
Recently, increasing number of HCV infection, involving the CNS, have been reported. Therefore, we recommend to check the HCV infection as a probable cause of various CNS neurologic disorders.
Figures and Tables
Fig. 1
FLAIR axial and contrast-enhanced T1-weighted axial images of brain MRI (A) and T2-weighted sagittal image of spine MRI on admission (B). (A) T2-high signal lesion with peripheral rim enhancement was noted in the brainstem. (B) Multifocal T2-high signal lesion on the brainstem and the cervical spinal cord. MRI, magnetic resonance imaging; FLAIR, fluid attenuated inversion recovery.
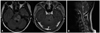
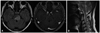
Fig. 2
FLAIR axial and contrast-enhanced T1-weighted axial images of brain MRI (A) and T2-weighted sagittal image of spine MRI (B) at follow-up two months later. (A) Remaining abnormal signal foci and no more enhanced lesion of the brainstem. (B) Nearly complete recovery of abnormal signal foci of the cervical spinal cord. MRI, magnetic resonance imaging; FLAIR, fluid attenuated inversion recovery.
References
1. Sacconi S, Salviati L, Merelli E. Acute disseminated encephalomyelitis associated with hepatitis C virus infection. Arch Neurol. 2001. 58:1679–1681.

2. Tan H, Kiliçaslan B, Onbaş O, Büyükavci M. Acute disseminated encephalomyelitis following hepatitis A virus infection. Pediatr Neurol. 2004. 30:207–209.

3. Alehan FK, Kahveci S, Uslu Y, Yildirim T, Yilmaz B. Acute disseminated encephalomyelitis associated with hepatitis A virus infection. Ann Trop Paediatr. 2004. 24:141–144.

4. Kang W, Shin EC. Clinical implications of chemokines in acute and chronic hepatitis C virus infection. Yonsei Med J. 2011. 52:871–878.

5. Dawson TM, Starkebaum G. Isolated central nervous system vasculitis associated with hepatitis C infection. J Rheumatol. 1999. 26:2273–2276.
6. Origgi L, Vanoli M, Carbone A, Grasso M, Scorza R. Central nervous system involvement in patients with HCV-related cryoglobulinemia. Am J Med Sci. 1998. 315:208–210.

7. Propst T, Propst A, Nachbauer K, Graziadei I, Willeit H, Margreiter R, et al. Papillitis and vasculitis of the arteria spinalis anterior as complications of hepatitis C reinfection after liver transplantation. Transpl Int. 1997. 10:234–237.

8. Bolay H, Söylemezoğlu F, Nurlu G, Tuncer S, Varli K. PCR detected hepatitis C virus genome in the brain of a case with progressive encephalomyelitis with rigidity. Clin Neurol Neurosurg. 1996. 98:305–308.

9. Annunziata P, Marroni M, Francisci D, Stagni G. Acute transverse myelitis and hepatitis C virus. Infez Med. 2005. 13:45–47.
10. De Carli DM, Pannebeker J, Pedro FL, Haygert CJ, Hertz E, Beck Mde O. Transverse myelitis associated to HCV infection. Braz J Infect Dis. 2009. 13:147–152.

11. Menge T, Hemmer B, Nessler S, Wiendl H, Neuhaus O, Hartung HP, et al. Acute disseminated encephalomyelitis: an update. Arch Neurol. 2005. 62:1673–1680.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download