Abstract
Purpose
To evaluate malignancy risk according to ultrasound (US) features and size change on follow-up US in mixed echoic thyroid nodules and to suggest management guidelines thereof.
Materials and Methods
Among patients who underwent US-guided fine needle aspiration biopsy, 316 mixed echoic nodules in 303 patients were included after excluding the patients with pure solid or cystic nodules or without further cytopathologic evaluation. We evaluated malignancy risk according to US features and changes in size and shape on follow-up US.
Results
The malignancy rate was 31.6% (6 of 19) for nodules with suspicious US features and 2.7% (8 of 297) for nodules without suspicious US features (p<0.001). Among 265 nodules with no suspicious US features and initial benign cytology, 15 nodules with suspicious US change and decreased size, 25 nodules with no suspicious US change and increased size, and 225 nodules with no suspicious US change and no change in size were observed on follow-up USs. The malignancy risk thereof was 0%, 0% and 0.4%, respectively (p=1.000).
Many thyroid nodules are incidentally detected in daily clinical practice, and they are present in nearly 50% of adults, increasing in prevalence with age;1 however, less than 5% of those nodules are malignant.2 Cystic thyroid nodules represent 15-53.8% of all surgically excised thyroid nodules,3-7 including true or simple thyroid cysts, with an epithelial lining, and mixed nodules with a predominantly fluid component.2,6 Among them, cystic thyroid nodules which are thyroid nodules characterized by the presence of both solid and cystic components and are referred to as "mixed echoic thyroid nodules",8 have been regarded as benign lesions that can be managed conservatively in the past.9,10 However, the percentage of malignancy among these mixed echoic nodules has been reported from 2% to 18%,2,4,6-8,11 however, these nodules often show non-diagnostic or inadequate cytologic result due to scant or no follicular cells, owing to the cystic portion,2,12 which is a challenge in managing these nodules with fine needle aspiration biopsy (FNAB).13
Repeated FNAB or surgery is advised for nodules that increase in size during follow-up.14-18 However, these mixed echoic nodules can increase in size upon follow-up ultrasound (US) by cystic degeneration,3,4 or sometimes they are decreased in size and demonstrate newly suspicious US features after FNAB by reduction of cystic portion.19 Until now, the management of mixed echoic thyroid nodules has not been firmly established, nor has a concrete study thereon been reported, to the best of our knowledge. The aim of this study was to evaluate the risk of malignancy, according to initial US features, US changes, or changes in size on follow-up US, in mixed echoic nodules and to suggest guidelines for managing these nodules.
Our institutional review board approved this retrospective observational study, and required neither patient approval, nor informed consent for review of patient images and records. However, informed consent for US-FNAB was obtained from all patients before conducting the procedure. From March 2006 to February 2007, 953 thyroid nodules in 868 consecutive patients underwent US-FNAB at our institution (a referral center) in Seoul, Korea. Among them, 580 nodules were excluded because they were either pure solid (n=576) or cystic nodules (n=4). Patients who met the following criteria upon review of their medical records, including cytologic and histopathologic results, were included in this study: 1) underwent thyroid surgery, 2) at least two benign cytologic results, and 3) benign results on initial cytologic results with no interval change or decrease in size at follow-up US after at least 12 months. Forty nodules were excluded owing to increase in size on follow-up US without further cytopathologic evaluation after demonstrating benign results on initial cytology. Seventeen nodules were also excluded because of lack of subsequent surgery after either papillary thyroid carcinoma (PTC) (n=2), suspicion of PTC (n=1), and indeterminate (n=1) or lack of subsequent surgery or follow-up FNAB after demonstrating inadequate results (n=13) on initial cytology. In total, this study included 316 mixed echoic nodules in 303 patients (Fig. 1). The mean age of the patients as a whole was 49.1 years (range, 16-80 years); the mean age of the male patients was 51.6 years (range, 21-72 years), and the mean age of the female patients was 49 years (range, 16-80 years). The mean size of the thyroid nodules was 20 mm (range, 4-76 mm).
US was performed with an 8- to 15-MHz linear array transducer (Acuson Sequoia; Siemens Medical Solutions, Mountain View, CA, USA), a 7- to 15-MHz linear array transducer (HDI 5000; Philips Medical Systems, Bothell, WA, USA), or a 5- to 12-MHz linear array transducer (iU22; Philips Medical Systems). Compound imaging was obtained in all images with HDI 5000 or iU22 machines. Real-time US imaging was performed by one of three radiologists with 11, 9, and 7 years of experiences in thyroid imaging who were aware of the patients' clinical background. US images were independently reviewed by each of two radiologists dedicated to thyroid imaging (K.J.Y. and S.Y.M. with 9 and 3 years of experience). Each thyroid nodule was described according to US features as devised in a previous report.20 We defined the cystic nodules, as designated in previous studies,2-6,13,21,22 as mixed echoic nodules comprising solid and cystic components.8 These mixed echoic nodules were evaluated based on their internal solid components. Echogenicity of the solid portion was categorized as hyperechoic (for nodules showing hyperechogenicity compared to the normal thyroid), isoechoic (for nodules showing isoechogenicity compared to the normal thyroid), hypoechoic (for nodules showing hypoechogenicity compared to the normal thyroid), or markedly hypoechoic (for nodules showing hypoechogenicity compared to the adjacent strap muscle). Margins of the nodules were classified as either well-circumscribed, microlobulated, or irregular. The degree of calcification was categorized as microcalcification (punctate echogenic foci of 1 mm or less), macrocalcification (punctate echogenic foci larger than 1 mm in size or eggshell calcification), mixed calcification (microcalcification combined with eggshell or macrocalcification) or negative (no calcification). Nodule shape was categorized as wider than tall or taller than wide. US groupings of thyroid nodules were classified as probably benign or suspiciously malignant in these nodules, using the sonographic criteria suggested by Kim, et al.20 Malignant sonographic characteristics were defined as microcalcification, irregular or microlobulated margins, markedly hypoechogenicity, and taller than wide shape. A suspiciously malignant nodule was defined as a nodule showing one or more of the suspicious findings presented above. If a nodule showed no suspicious features, it was classified as probably benign. We evaluated the changes in size and shape of the thyroid nodules on follow-up US in this study. We categorized nodules as being increased in size when its maximum diameter increased by more than 3 mm,21 irrespective of the initial size of the nodule. Also, we defined newly developed suspicious US features as a transition from being probably benign on initial US to suspiciously malignant on follow-up US after FNAB.
FNAB was performed by a single radiologist after US evaluations of thyroid glands for either thyroid nodules with suspicious US features or the largest nodule present if no suspicious malignant US features were detected. FNAB was conducted with the patient in the supine position with the neck extended using a 23-gauge needle (Korea Vaccine, Seoul, Korea) attached to a 20 mL disposable plastic syringe (Kovax-Syringe, Korea Vaccine) and an aspirator under US guidance with a free hand technique. Each lesion was aspirated at least twice. All cystic fluid was aspirated as much as possible from the cystic portion of the nodules and then FNAB was performed on the solid portion of the nodules. If complete aspiration was not possible in the thick cystic contents of thyroid nodules, FNAB was directly conducted on the solid portion of the mixed echoic nodules. Materials obtained from FNAB were smeared on glass slides, which were immediately placed in 95% alcohol for Papanicolau staining, and the remaining material in the syringe was rinsed in saline for processing as a cell block. A cytologist was not on site during the biopsy. Cytology samples were sent to the pathology department and the results were reported by 1 of 5 cytopathologists in an official report form. Additional special staining was performed on a case-by-case basis following the discretion of the cytopathologist. Cytological results were classified as into one of the following two categories: "adequate" or "inadequate" for interpretation. A specimen was considered as "adequate" if there was a minimum of six groupings of well-preserved thyroid cells consisting of at least ten cells per group.23 The "adequate" group was divided into four subgroups: "benign", "indeterminate", "suspicious for PTC", and "PTC". "Benign" cytology included colloid nodules, nodular hyperplasia, lymphocytic thyroiditis, Graves' disease, and postpartum thyroiditis. "Indeterminate" cytology included follicular and Hürthle cell neoplasm. "Suspicious for PTC" cytologic results were defined when the specimen exhibited cytological atypia, but showed insufficient cellularity for definite diagnosis of PTC. Cytologic results were designated as "PTC" when specimen revealed abundant cells with definite cytological features of malignancy.24
Malignant nodules were confirmed by surgery or FNAB, while benign nodules were confirmed as benign if they were classified as benign at both initial and follow-up FNAB, upon surgery, or if the nodules either decreased or showed no interval change at follow-up US at least 12 months (mean, 30.7 months; range, 12-58 months) after being classified as benign on FNAB.
We used a χ2-test to determine differences between malignant and benign nodules according to categorical variables. We also evaluated the differences between malignant and benign nodules with continuous variables using an independent two sample t-test. Statistical significance was assumed when the p-value was less than 0.05. All reported p-values were two-sided. Statistical analyses were performed by using SAS software version 9.1 (SAS Institute Inc., Cary, NC, USA).
Of the 316 thyroid nodules, 14 (4.4%) were malignant and 302 (95.6%) were benign. The mean size of the malignant nodules were 19.1±13.7 mm, showing no significant differences to the benign nodules (20.0±12.2 mm) (p=0.773). The mean age (49.6±11.7 years) of the patients with benign nodules was significantly older than that (40.5±12.5 years, p=0.006) of the patients with malignant nodules. There were no significant differences between benign and malignant nodules according to gender (p=0.337).
Among total 316 nodules, there were 298 benign results: 8 suspicious for PTC, 4 PTC and 6 inadequate results according to follow-up US or cytopathologic confirmation. Thirty-seven nodules (11.7%) underwent surgery without undergoing follow-up FNAB or US, for the following reasons: cytologic results suspicious for PTC (n=8) and PTC (n=4), large size nodules with inadequate (n=2) or benign (n=14) cytology, and upon patient request (n=9). Among those who underwent surgery, 13 were malignant and the remaining 24 were benign.
The remaining 279 nodules underwent follow-up FNAB or follow-up US after more than 12 months. The mean size of the initial thyroid nodules was 19.7±11.7 mm (range, 4-76 mm) and the mean size of the nodules after follow-up US or FNAB was 16.9±11.5 mm (range, 2-81 mm) (p<0.001) in these 279 nodules. Follow-up FNAB was performed in 127 nodules. The grounds for follow-up FNAB were: clinician or patient request (n=71), growth at follow-up US (n=26), suspicious US features (n=25) and newly developed suspicious features with decreased size at follow-up US (n=5). The mean interval between initial and follow-up FNAB was 23.3 months (range, 3-51 months). Follow-up US was performed in 152 nodules and the mean interval between initial and follow-up US was 30.9 months (range, 10-58 months).
Of the 316 nodules, there were 19 (6%) nodules with suspicious US features and 297 (94%) nodules with no suspicious US features. The rate of malignancy was 31.6% (6 of 19) for thyroid nodules with suspicious US features and 2.7% (8 of 297 nodules) for those with no suspicious US features. There was significant difference between US grouping of thyroid nodules and the rate of malignancy (p<0.001).
Among thyroid nodules with initially benign cytology (n=298), the malignancy rate of thyroid nodules assessed as having suspicious US features was 7.7% (1/13) while those with no suspicious US features was 1.1% (3/285) (p=0.164). Of thyroid nodules (n=275) with initial benign cytology that underwent follow-up US or FNAB, there were 26 nodules of increased size with a mean size of 5.8 mm (from range of 8-76 mm to the range of 12-81 mm), 156 nodules with no change in size and 93 nodules with decreased size. There were no thyroid nodules observed with suspicious change and growth. Of these nodules (n=275) with initial benign cytology that underwent follow-up US or FNAB, 265 thyroid nodules had no suspicious US features, while 10 had suspicious US features. Among the 265 thyroid nodules with no suspicious US features and benign results on initial cytology, there were 15 nodules with suspicious US change and decreased size (Fig. 2), 25 with no suspicious US change and increased size (Fig. 3) and 225 with no suspicious US change as well as no change or decrease in size on follow-up US. The likelihood of a nodule being benign based on standard reference was 100% (15 of 15) in the group of nodules with suspicious US change and decreased size, 100% (25 of 25) in the group with no change in shape and increased size, and 99.6% in the group with no change in shape and size or decreased size (224 of 225), respectively. Therefore, the risk of malignancy was 0%, 0% and 0.4%, respectively, showing no statistical difference among the three groups (p=1.000).
Of six thyroid nodules with inadequate cytology (two confirmed by surgery and four by repeat FNAB), there was no malignancy. Five of them showed no suspicious US features, and one had suspicious US features. Four nodules showed no suspicious US change with decreased size (n=2) and no change in size (n=2). The remaining two nodules underwent surgery without follow-up.
Mixed echoic nodules represent 15-53.8% of all surgical excised thyroid nodules.3-7 They have been regarded as benign nodules with a rare risk of malignancy and can be managed conservatively.9,10 However, the incidence of malignancy in these mixed echoic nodules are reported as 2% to 18%.2,4,6-8,11,25 Accordingly, the concrete assessment of the management of these nodules is necessary. Discriminating between benign and malignant thyroid nodules, many reports17,20,26-33 described suspicious US features such as irregular or microlobulated margins,17,20,30,34-38 hypoechogenicity,17,20,29,30,34,37,38 marked hypoechogenicity,20,29 taller than wide shape,17,20,39 microcalcification,17,20,30,36-38,40-42 solid composition17,36-38,43 and intratumoral vascularity.17,30,34,43 US findings are also important for the discrimination of mixed echoic thyroid nodules. The eccentric location of a solid mural nodule,8,17,25,44 eccentric configuration with an acute angle of the solid portion,25 its vascularity of the solid portion,40 and the presence of microcalcification8,25,44 have been reported as suspicious US features in mixed echoic nodules suggestive of PTC.
We intended to evaluate the risk of malignancy according to US features and US change or size change on follow-up US in the mixed echoic nodules. The rate of malignancy was 31.6% for thyroid nodules with suspicious US features, significantly higher in comparison to 2.7% nodules with no suspicious US features in this study. These results indicate the importance of US features in differentiating benign from malignant among thyroid nodules, and the results herein were consistent with the results of previous studies.24,45 Among thyroid nodules with initially benign cytology (n=298), the malignancy rate (7.7%, 1/13) of thyroid nodules with suspicious US features was higher than that (1.1%, 3/285) of thyroid nodules with no suspicious US features even though there was no significant difference (p=0.1641). Therefore, mixed echoic nodules with suspicious features on initial US can be an indication for follow-up FNAB like solid nodules.24,45
In this study, we also divided nodules into three groups according to changes in the shape and size of the thyroid nodules, as follow-up FNAB was previously recommended for thyroid nodules with substantial growth on follow-up US, even if thyroid nodules were diagnosed as benign on prior cytology.16-18 However, the scientific evidence thereof is not sufficient. Because many benign nodules can grow as mixed echoic nodules do due to cystic degeneration,3,4 nodular growth itself might not be a pathognomonic sign of malignancy. In this study, among mixed echoic nodules with initial benign cytology (n=275), 26 (9.4%) thyroid nodules showed growth on follow-up and the rate of malignancy was 0%.
Recent studies reported that mixed echoic nodules can also show suspicious US findings after FNAB by the reduction of cystic contents; however, these were proven as benign on follow-up cytology. Therefore, correlation with past FNAB history can prevent future unnecessary FNAB and follow-up US might be better recommended rather than to follow-up FNAB.19 In this study, we divided nodules into three groups according to the changes in US features as well as the size of thyroid nodules on follow-up US. The risk of malignancy was 0% in the group with suspicious US change and decreased size, 0% in the group with no change in shape but increased size, and 0.4% in the group with no change in shape and size or no change in shape but decreased size, respectively, without statistical significance. Therefore, in mixed echoic nodules with benign results on cytology and with no suspicious US features, follow-up US may be sufficient in the management of these nodules, even if they showed growth or changes of newly developed suspicious US features after FNAB with decreased size on follow-up US.
Most studies have focused on solid thyroid nodules, but few have reported the follow-up of mixed echoic nodules.2,4,6,8,11,25 Some investigators reported that these mixed echoic thyroid nodules can show non-diagnostic or inadequate cytologic results due to scant or no follicular cells of cystic portion.2,12 Frates, et al.17 suggested the more cystic a nodule was, the lower the likelihood of cancer with statistical significance. Lower malignancy rate was also reported in thyroid nodules of inadequate cytology without suspicious US features, compared with those with suspicious US features, especially, in cystic nodules.46 Of the six thyroid nodules with inadequate cytology in this study, five of them showed no suspicious US features, and one had suspicious US features, and there was no malignant lesion, consistent with other report.46
There are several limitations to our study. First, there was relatively small number of patients with mixed echoic nodules among the total study population and only a small number of nodules (n=37, 11.7%) was underwent surgery. Also, the remaining thyroid nodules which were included in this study were underwent follow-up US at least 12 months or FNAB without surgery as a reference standard. A somewhat short follow-up period of 12 months was also another limitation, and the possibility of false negative and false positive results exists. Second, this study was a retrospective one, thus color Doppler images of the sold portion of mixed echoic nodules were not evaluated. Also, this study contemplated the entire nodule instead of only the solid portion as representative of nodular growth. The revised American Thyroid Association guidelines suggested that repeat FNAB should be based upon growth of the solid component, especially for, mixed echoic nodules;16 the differentiation of solid and cystic components in mixed echoic nodules is challenging since the solid and cystic components are intermingled. Thus, the measurement of growth of the solid component only is difficult. To resolve this issue, 3D US might be helpful. Lastly, in this study, because there was no combination of thyroid nodules classified as benign on cytology that showed both no changes in size or growth with benign nodules that showed newly developed suspicious changes on follow-up US, the larger study group that can fulfill these combinations will be necessary.
In conclusion, the management of mixed echoic nodules depends on US features. The nodules with no suspicious US features and benign cytology can be followed up using US, as they revealed very low rate of malignancy, even if they showed growth on follow-up US.
Figures and Tables
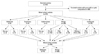 | Fig. 1Diagram of the study population. non-op, without operation; Op, with operation; FNA, fine needle aspiration. |
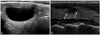 | Fig. 2A 51-year-old woman with a thyroid nodule in the right thyroid on US. Initial FNAB revealed the nodule to be benign on cytology, and follow-up FNAB after 1.5 year showed the same cytologic result. (A) Initial US shows a mixed echoic nodule (>50% cystic) (arrows). (B) On follow-up US, this nodule showed decreased size and suspicious US changes including hypoechoic and microlobulated margins and taller-than wide shape (arrows). US, ultrasound; FNAB, fine needle aspiration biopsy. |
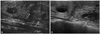 | Fig. 3A 56-year-old woman with a thyroid nodule in the right thyroid on US. Initial FNAB revealed the nodule to be benign on cytology, and follow-up FNAB after 3 year showed the same cytologic result. (A) Initial US shows a mixed echoic nodule (< 50% cystic) (arrows). (B) On follow-up US, this nodule showed increased size without change in shape (arrows). US, ultrasound; FNAB, fine needle aspiration biopsy. |
References
2. Choi KU, Kim JY, Park DY, Lee CH, Sol MY, Han KT, et al. Recommendations for the management of cystic thyroid nodules. ANZ J Surg. 2005. 75:537–541.

3. de los Santos ET, Keyhani-Rofagha S, Cunningham JJ, Mazzaferri EL. Cystic thyroid nodules. The dilemma of malignant lesions. Arch Intern Med. 1990. 150:1422–1427.

4. McHenry CR, Slusarczyk SJ, Khiyami A. Recommendations for management of cystic thyroid disease. Surgery. 1999. 126:1167–1171.

5. Meko JB, Norton JA. Large cystic/solid thyroid nodules: a potential false-negative fine-needle aspiration. Surgery. 1995. 118:996–1003.

6. Bellantone R, Lombardi CP, Raffaelli M, Traini E, De Crea C, Rossi ED, et al. Management of cystic or predominantly cystic thyroid nodules: the role of ultrasound-guided fine-needle aspiration biopsy. Thyroid. 2004. 14:43–47.

7. Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf). 2004. 60:21–28.

8. Lee MJ, Kim EK, Kwak JY, Kim MJ. Partially cystic thyroid nodules on ultrasound: probability of malignancy and sonographic differentiation. Thyroid. 2009. 19:341–346.

9. Crile G Jr. Treatment of thyroid cysts by aspiration. Surgery. 1966. 59:210–212.
10. Sarda AK, Bal S, Dutta Gupta S, Kapur MM. Diagnosis and treatment of cystic disease of the thyroid by aspiration. Surgery. 1988. 103:593–596.
11. Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006. 91:3411–3417.

12. Alexander EK, Heering JP, Benson CB, Frates MC, Doubilet PM, Cibas ES, et al. Assessment of nondiagnostic ultrasound-guided fine needle aspirations of thyroid nodules. J Clin Endocrinol Metab. 2002. 87:4924–4927.

13. Braga M, Cavalcanti TC, Collaço LM, Graf H. Efficacy of ultrasound-guided fine-needle aspiration biopsy in the diagnosis of complex thyroid nodules. J Clin Endocrinol Metab. 2001. 86:4089–4091.

14. Kuma K, Matsuzuka F, Yokozawa T, Miyauchi A, Sugawara M. Fate of untreated benign thyroid nodules: results of long-term follow-up. World J Surg. 1994. 18:495–498.

15. Singer PA, Cooper DS, Daniels GH, Ladenson PW, Greenspan FS, Levy EG, et al. American Thyroid Association. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. Arch Intern Med. 1996. 156:2165–2172.

16. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009. 19:1167–1214.

17. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005. 237:794–800.

18. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010. 16:Suppl 1. 1–43.

19. Koo JH, Shin JH, Han BK, Ko EY, Kang SS. Cystic thyroid nodules after aspiration mimicking malignancy: sonographic characteristics. J Ultrasound Med. 2010. 29:1415–1421.
20. Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002. 178:687–691.

21. Alexander EK, Hurwitz S, Heering JP, Benson CB, Frates MC, Doubilet PM, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med. 2003. 138:315–318.

22. Müller N, Cooperberg PL, Suen KC, Thorson SC. Needle aspiration biopsy in cystic papillary carcinoma of the thyroid. AJR Am J Roentgenol. 1985. 144:251–253.

23. Gharib H, Papini E, Valcavi R, Baskin HJ, Crescenzi A, Dottorini ME, et al. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006. 12:63–102.
24. Kwak JY, Kim EK, Kim HJ, Kim MJ, Son EJ, Moon HJ. How to combine ultrasound and cytological information in decision making about thyroid nodules. Eur Radiol. 2009. 19:1923–1931.

25. Kim DW, Lee EJ, In HS, Kim SJ. Sonographic differentiation of partially cystic thyroid nodules: a prospective study. AJNR Am J Neuroradiol. 2010. 31:1961–1966.

26. Cappelli C, Castellano M, Pirola I, Gandossi E, De Martino E, Cumetti D, et al. Thyroid nodule shape suggests malignancy. Eur J Endocrinol. 2006. 155:27–31.

27. Iannuccilli JD, Cronan JJ, Monchik JM. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med. 2004. 23:1455–1464.

28. Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011. 12:1–14.

29. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008. 247:762–770.

30. Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002. 87:1941–1946.

31. Tae HJ, Lim DJ, Baek KH, Park WC, Lee YS, Choi JE, et al. Diagnostic value of ultrasonography to distinguish between benign and malignant lesions in the management of thyroid nodules. Thyroid. 2007. 17:461–466.

32. Alexander EK, Marqusee E, Orcutt J, Benson CB, Frates MC, Doubilet PM, et al. Thyroid nodule shape and prediction of malignancy. Thyroid. 2004. 14:953–958.

33. Lee MJ, Hong SW, Chung WY, Kwak JY, Kim MJ, Kim EK. Cytological results of ultrasound-guided fine-needle aspiration cytology for thyroid nodules: emphasis on correlation with sonographic findings. Yonsei Med J. 2011. 52:838–844.

34. Cappelli C, Castellano M, Pirola I, Cumetti D, Agosti B, Gandossi E, et al. The predictive value of ultrasound findings in the management of thyroid nodules. QJM. 2007. 100:29–35.

35. Ito Y, Kobayashi K, Tomoda C, Uruno T, Takamura Y, Miya A, et al. Ill-defined edge on ultrasonographic examination can be a marker of aggressive characteristic of papillary thyroid microcarcinoma. World J Surg. 2005. 29:1007–1011.

36. Kang HW, No JH, Chung JH, Min YK, Lee MS, Lee MK, et al. Prevalence, clinical and ultrasonographic characteristics of thyroid incidentalomas. Thyroid. 2004. 14:29–33.

37. Koike E, Noguchi S, Yamashita H, Murakami T, Ohshima A, Kawamoto H, et al. Ultrasonographic characteristics of thyroid nodules: prediction of malignancy. Arch Surg. 2001. 136:334–337.

38. Peccin S, de Castsro JA, Furlanetto TW, Furtado AP, Brasil BA, Czepielewski MA. Ultrasonography: is it useful in the diagnosis of cancer in thyroid nodules? J Endocrinol Invest. 2002. 25:39–43.

39. Cappelli C, Pirola I, Cumetti D, Micheletti L, Tironi A, Gandossi E, et al. Is the anteroposterior and transverse diameter ratio of nonpalpable thyroid nodules a sonographic criteria for recommending fine-needle aspiration cytology? Clin Endocrinol (Oxf). 2005. 63:689–693.

40. Chan BK, Desser TS, McDougall IR, Weigel RJ, Jeffrey RB Jr. Common and uncommon sonographic features of papillary thyroid carcinoma. J Ultrasound Med. 2003. 22:1083–1090.

41. Khoo ML, Asa SL, Witterick IJ, Freeman JL. Thyroid calcification and its association with thyroid carcinoma. Head Neck. 2002. 24:651–655.

42. Rago T, Vitti P, Chiovato L, Mazzeo S, De Liperi A, Miccoli P, et al. Role of conventional ultrasonography and color flow-doppler sonography in predicting malignancy in 'cold' thyroid nodules. Eur J Endocrinol. 1998. 138:41–46.

43. Frates MC, Benson CB, Doubilet PM, Cibas ES, Marqusee E. Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules? J Ultrasound Med. 2003. 22:127–131.

44. Hatabu H, Kasagi K, Yamamoto K, Iida Y, Misaki T, Hidaka A, et al. Cystic papillary carcinoma of the thyroid gland: a new sonographic sign. Clin Radiol. 1991. 43:121–124.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download