Abstract
Purpose
Hybrid therapy with catheter ablation of the cavo-tricuspid isthmus (CTI) and continuation of anti-arrhythmic drugs (AAD), or electrical cardioversion with AADs might be alternative treatments for patients with persistent atrial fibrillation (AF). The goal of study was to assess the long term success rate of hybrid therapy for persistent AF compared to antiarrhythmic medication therapy after electrical cardioversion and identify the independent risk factors associated with recurrence after hybrid therapy.
Materials and Methods
A total of 32 patients with persistent AF who developed atrial flutter after the administration of a class Ic or III anti-arrhythmic drug were enrolled. This group was compared with a group (33 patients) who underwent cardioversion and received direct current cardioversion with AADs. Baseline data were collected, and electrocardiogram and symptom driven Holter monitoring were performed every 2-4 months.
Results
There was no significant difference in the baseline characteristics between the groups. The 12 month atrial arrhythmia free survival was better in the hybrid group, 49.0% vs. 33.1%, p=0.048. However, during a mean 55.7+/-43.0 months of follow up, the improved survival rate regressed (p=0.25). A larger left atrium size was an independent risk factor for the recurrence of AF after adjusting for confounding factors.
Conclusion
Despite favorable outcome during 12 month, the CTI block with AADs showed outcomes similar to AAD therapy after electrical cardioversion over a 12 month follow up period. Minimal substrate modification with AADs might be an alternative treatment for persistent AF with minimal atrial remodeling.
Catheter ablation is considered standard therapy by current treatment guidelines1 for patients with symptomatic paroxysmal atrial fibrillation (AF) who do not respond to single antiarrhythmic drug therapy (AAD). However, the effectiveness of catheter ablation is low in patients with persistent AF despite prolongation of the complex procedure.2-4 Therefore, the mainstay of therapy for these patients has been long-term AAD even though persistent AF is poorly controlled by AAD therapy. Furthermore, in the latest guideline,1 longterm AAD therapy is indicated in patients who have troublesome symptoms related to paroxysmal AF or recurrent AF after cardioversion who can tolerate antiarrhythmic drugs and have a good chance of remaining in sinus rhythm over an extended period (e.g., young patients without organic heart disease or hypertension, a short duration of AF, and normal LA size). In some patients, AF evolves to typical atrial flutter after the initiation of anti-arrhythmic drug therapy. Previously, catheter ablation of the inferior vena cava-tricuspid annulus isthmus along with continuation of anti-arrhythmic medication therapy (hybrid therapy) has been shown to be effective treatment for anti-arrhythmic drug-induced atrial flutter.5-8 Although a high initial success rate establishing a stable sinus rhythm has been described in patients with hybrid therapy, post-ablation atrial fibrillation can recur during follow-up, despite the continuation of anti-arrhythmic medication. Most patients have good immediate and intermediate outcomes with hybrid therapy in regard to their paroxysmal AF. Hybrid therapy may be effective in patients with conversion to typical atrial flutter occurring in 10 to 15% of patients with persistent AF who are undergoing antiarrhythmic medication. However, the long term clinical outcome of hybrid therapy in patients with chronic persistent AF has not yet been evaluated and the risk factors associated with the recurrence of AF have not been identified. Furthermore, there is few data comparing hybrid therapy and drug therapy after electrical cardioversion.
Therefore, the goal of this study was to assess the long term success rate of hybrid therapy for persistent AF compared to sole antiarrhythmic medication therapy after electrical cardioversion and identify the independent risk factors associated with recurrence after hybrid therapy in patients with persistent AF.
Persistent AF was defined as AF lasting more than 7 days. It was considered to be highly likely if two electrocardiographic studies, including 24 hour Holter monitoring, were without an episode of sinus rhythm. From January 1999 to December 2007, 250 patients underwent catheter ablation for atrial flutter at our institution. Among these patients, we identified 32 patients who developed atrial flutter after anti-arrhythmic drug therapy for chronic persistent AF without evidence of pre-drug atrial flutter (hybrid group). The clinical outcomes and echocardiographic data from this group were compared to those of 33 persistent AF patients that underwent successful electrical cardioversion with antiarrhythmics during the same period electrocardioversion group (EC group).
Demographic, clinical and procedural variables were collected retrospectively by review of the medical records. All patients underwent non-invasive cardiac examination including M-mode and two dimensional echocardiography with color Doppler flow analysis. Valvular heart disease was defined as existence of moderate to severe regurgitation or stenosis of valve in echocardiography. Left atrial enlargement was defined as a left atrial anterior-posterior diameter more than 44 mm.
This study received approval from the institutional ethics committee, and the procedures followed were in accordance with the institutional guideline.
All patients in the hybrid group received electrophysiological studies and radiofrequency ablation after informed consent was obtained. Antiarrhythmics were continued. Three multipolar electrode catheters were inserted percutaneously into the left femoral vein. Two quadripolar catheters (2-5-2 mm spacing, DAIG, St Jude Medical Inc., St. Paul, MN, USA) were positioned at the his bundle and right atrium. A decapolar catheter (2-5-2 mm spacing, DAIG, St Jude Medical Inc., St. Paul, MN, USA) was positioned in the coronary sinus via the left subclavian vein. Activation sequence of atrial flutter or entrapment mapping was assessed by a 20-pole electrode halo catheter (2-10-2 mm spacing, Biosense-Webster, Diamond Bar, CA, USA). The position of the catheters was checked in the 45° left anterior oblique view and the 30° right anterior oblique view. A deflectable 5 mm-tipped quadripolar catheter or irrigation catheter was used as the ablation catheter. The electrophysiological definition of isthmus dependent atrial flutter was demonstrated by the standard criteria9 for entrainment mapping. A 500 kHz radiofrequency (RF) ablation unit (Stockert, Biosense-webster, Diamond Bar, CA, USA) was used for the ablation. RF ablation started at the ventricular side of inferolateral tricuspid annulus and the end point was bidirectional block of isthmic conduction.
Delivery of impulses was guided by impedance control. A bidirectional isthmus block was demonstrated by pacing technique which was performed at coronary sinus ostium and the low lateral right atrium. The line of block was reevaluated 30 min after the catheter ablation.
Electrical cardioversion was performed after 4-6 weeks of anticoagulation with warfarin or when LA thrombi were not seen on the transesophageal echocardiography. R wave synchronized direct current (DC) shock with a monophasic or biphasic waveform was delivered during sedation induced by intravenous pentothal sodium (1.5 mg/kg). One defibrillator pad, with a 10-cm diameter, was placed at the second intercostal space on the right side parasternally and the other was placed at a left-sided lateral position along the midaxillary line. The cardioversion procedure started with 100 J (biphasic) or 200 J (monophasic), followed by 200 J and 300 J, until the restoration of sinus rhythm.
Demographic, clinical and procedural variables were collected retrospectively by review of the medical records. All patients in both groups were instructed to continue the anti-arrhythmic medications. Most patients received warfarin to maintain an International Normalized Ratio of 2.0-3.0 for at least one month after the ablation. In some patients who had no indications for systemic anticoagulation, warfarin was changed to aspirin 100 mg daily after 1-2 months of anticoagulation. All patients had regular follow-up with standard 12 lead electrocardiogram (ECG) in the out-patient clinic every 2-4 months or Holter monitoring if the patient was symptomatic. Patients with palpitations or symptoms suggestive of atrial fibrillation or atrial flutter underwent ambulatory 24-hour Holter monitoring. Recurrent atrial fibrillation was diagnosed when documented by ECG or Holter monitoring. Patients were instructed to obtain an ECG at the nearest hospital in cases where symptomatic palpitation developed. Patients were followed for a mean period of 55.7+/-43.0 months (up to 7 years) after catheter ablation or electrical cardioversion. The follow-up end point was recurrence of atrial fibrillation or flutter.
Continuous variables are expressed as mean±SD, and categorical variables as percents. The unpaired Student t-test and χ2 analysis were used to compare continuous and categorical variables, respectively. The 95% confidence interval (CI) was determined on the basis of the normal distribution of variables. Kaplan-Meier estimates were used to determine overall survival and event-free survival for both groups. Comparison between the groups was performed using the log-rank test. Cox regression hazard analysis was used to identify independent correlates of long-term event-free survival. All analyses were performed using SPSS software version 11.5 (SPSS Inc., Chicago, IL, USA).
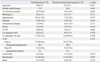
The baseline characteristics of both groups of patients are listed in Table 1. When compared with the cardioversion group, the hybrid group included more patients with diabetes (28.1% vs. 9.1%; p=0.018). However, there was no difference in age, gender, body mass index, or valvular heart disease. No significant difference was found with regard to the periprocedural medications such as antiarrhythmics, angiotensin converting enzyme inhibitors/angiotensin receptor blockers, beta blockers and calcium channel blockers between the two groups. The pre-procedural echocardiographic parameters showed that the left ventricular dimension and left ventricular systolic function were within normal range and there was no difference between the groups. The left atrial size, measured by the anterior-posterior distance in the parasternal long axis view, was not statistically different; patients with more than 44 mm left atrial anterior-posterior diameter were more common in the hybrid group, but the difference was not statistically significant (53.1% vs. 48.5%, p=0.68).
Four patients (12.5%) in the hybrid group presented with atypical atrial flutter on the 12 lead ECG and showed clockwise atrial activation sequences. Atypical atrial flutter was treated with the catheter ablation procedure. It was cavo-tricuspid isthmus (CTI) dependent and successfully ablated with CTI block; 28 patients (87.5%) had common atrial flutter and all atrial flutters were cavo-tricuspid isthmus dependent as proven by entrapment mapping and terminated by CTI block. The mean cycle length of the atrial flutter on the electrophysiological studies was 325.8+/-127.6 ms. All patients achieved complete bidirectional isthmus block and non-inducibility of atrial flutter, which was the end point of the catheter ablation. The procedure time, flouro time and RF time were: 87.3±42.6 min, 32.8±12.6 min, and 1,034.7±698.2 sec, respectively. All patients in the EC group achieved a sinus rhythm after cardioversion without complication.
The mean follow-up was 67+/-33 months and 101+/-38 months in the hybrid group and EC group, respectively. During the follow-up period, recurrent atrial fibrillation or atrial tachycardia/flutter were developed in all patients. In the hybrid group, typical atrial flutter recurred in 2 patients, and atrial tachycardia or atypical atrial flutter developed in 13 patients. Seventeen patients experienced atrial fibrillation. In the EC group, atrial fibrillation recurred in the greater part of patients, and atrial tachycardia or atypical atrial flutter developed in 4 patients. Typical atrial flutter developed in three patients. By Kaplan-Meier estimates, the 12 month AF free-survival was better in the hybrid group (12 months event-free survival 49.0% for the hybrid group patients and 33.1% for the EC group patients, p=0.048) (Fig. 1). However, during a mean of 55.7+/-43.0 months of follow up, this better survival rate regressed (5 year event-free survival 24.0% for the hybrid group patients and 16.5% for EC group patients, p=0.28) (Fig. 2). On the multivariate analysis, the type of procedure (Hybrid or EC) was not a statistically significant indicator of the long-term outcome. The preprocedural LA size was the only independent risk factor for the recurrence of atrial arrhythmia after adjusting for age, gender, diabetes, hypertension, stroke and ejection fraction (odd ratio 2.5, CI of 95% 1.494-3.66, p=0.02) (Table 2).
In comparison with paroxysmal AF, persistent AF has a different underlying pathophysiological mechanism.10,11 There are definite triggers and drivers of paroxysmal AF that cannot maintain long term fibrillatory activity. Paroxysmal AF is frequently triggered by ectopic beats from the muscular sleeve extending from the left atrium into the pulmonary veins (PV). Therefore, the target of treatment is the elimination of these triggers and drivers, such as PV isolation. However, in patients with persistent AF, the triggers and drivers are located at unusual sites, and the fibrillatory activity is independent. The remodeled atria act as substrate and can perpetuate fibrillatory waves and independently maintain chaotic electrical activity. Therefore, for restoration of sinus rhythm, more prolonged and highly sophisticated procedures are required for the treatment of patients with chronic persistent AF, and the efficacy of catheter ablation is lower than in paroxysmal AF, with a significant increase in the associated risk of procedural complications.
Several alternative strategies have been considered for these patients. Hybrid therapy, a combined method of pharmacological and non-pharmacological therapy, is one of the alternatives; it can be used for certain patients with atrial flutter that is transformed from atrial fibrillation after initiation of drug treatment. A previous study indicates that the incidence of drug-related atrial flutter is up to 12.8% among patients that had received classic anti-arrhythmic drug therapy for atrial fibrillation.6,12 In these patients, hybrid therapy has a good outcome without significant complications. Different studies have documented 7% up to 42% AF recurrence rate during 12 month to 2 year follow up.13-15 However, these studies included more paroxysmal patients than persistent and detected AF recurrence with symptom driven ECG recording. In contrast to previous studies, the present study included only patients with chronic persistent AF which continued overall 50 months, and showed a higher recurrence rate of AF by regular ECG follow up and symptom-driven Holter monitoring. However, the 12 month AF free-survival rate was comparable to that of previous AF ablation studies in patients with chronic persistent AF.16 Furthermore, a worldwide survey revealed that the success rate of AF ablation, including patients with paroxysmal and persistent AF, varied according to the volume of cases undergoing the procedure per center, and that the major complication rate was 5.9%, including early deaths.17 The results of this study and the evidence from previous studies7,8 suggest that hybrid treatment is a reasonable approach for patients with atrial flutter that evolved from persistent AF.
In contrast to hybrid therapy, sole antiarrhythmic medication therapy after direct current (DC) cardioversion had a poorer outcome over the 12 months of follow up. Previously, the PAFAC trial reported a 65-67% rate of AF recurrence after DC cardioversion.18 In the trial, sotalol and quinidine were used as antiarrhythmic medications, and the clinical outcome was comparable with the use of flecainide, propafenone or amiodarone in our present study. However, during a mean of 55.7+/-43.0 months of follow up, the benefit of hybrid therapy regressed and was not significant compared to single drug therapy (p=0.25). Reithmann, et al.19 reported that the presence of pre-ablation episodes of atrial fibrillation, in patients taking anti-arrhythmic medication, and a decreased left ventricular ejection fraction were significant and independent predictors of post-ablation atrial fibrillation. In our study, the only independent risk factor for recurrence of AF was the pre-ablative LA size. The likely explanation for this result is atrial electrical and structural remodeling. Electrical remodeling accompanied by structural remodeling acts as substrate, and more modification of the substrate is required to restore sinus rhythm.
Stabile, et al.20 reported that single drug therapy in patients with atrial flutter, after flecainide infusion, showed an 80% AF recurrence rate over 12 months period, suggesting that a CTI block plays a critical role in preventing AF recurrence in patients with drug-induced atrial flutter. A previous randomized study on the effects of CTI ablation in patients with typical atrial flutter and atrial fibrillation revealed that CTI block reduced only the early postablation recurrence of arrhythmias, and that pulmonary vein isolation might be sufficient to control both arrhythmias.21 However, in drug-induced atrial flutter, the CTI block had a different significant meaning. In an animal model, the spontaneous transformation of AF was related to the development of a line of functional block between the vena cavae.22 This process was enhanced by the administration of antiarrhythmic medication. Recently, Waldo and Feld23 described an inter-relationship between atrial fibrillation and atrial flutter; a transient period preceding atrial fibrillation prior to atrial flutter is present in humans. During the short period of AF, a functional line of block developed and fibrillatory waves evolved to regular macro-reentrant waves. The antiarrhythmic medications with sodium channel blocking properties have been associated with significant conversion of AF to atrial flutter, suggesting that these drugs make it possible for a functional line of block to form between the vena cavae during the initial atrial fibrillation rhythm. The depression of atrial conduction velocity with a consecutive transformation of conduction delay into a conduction block, caused by antiarrhythmic medications, prevents the simultaneous occurrence of the multiple reentrant circuits necessary for the perpetuation of atrial fibrillation, resulting in a single atrial flutter reentrant circuit with an area of slow conduction. Therefore, block of the slow conduction of atrial flutter, with continuation of antiarrhythmic medication, can be an alternative treatment for patients with persistent AF, especially in cases with minimal atrial remodeling. However, in patients with persistent AF and severe atrial remodeling, rhythm control could not be maintained, and AF recurrence frequently occurred. Therefore, rate control without antiarrhythmics might be a more reasonable approach for long term treatment in these patients. Preprocedural evaluation of patients, including imaging study, is essential to choose the type of treatement and predict the prognosis of the clinical outcome.
This study was not planned as a randomized prospective study. Although all data on the hybrid therapy performed previously were collected retrospectively, the documented atrial flutter was induced after anti-arrhythmic drug therapy for atrial fibrillation. In addition, the incidence of atrial flutter caused by atrial fibrillation after anti-arrhythmic drug use could not be estimated. Although the general incidence of atrial flutter transformed from atrial fibrillation has been reported to be 10 to 20%, this data were collected, based on a Western study; there are no data on Asian populations. Even though standard 12 lead ECG was regularly performed regardless of symptom, Holter and event monitors were only available for symptomatic recurrences; therefore, it is possible that some asymptomatic recurrences of atrial fibrillation were missed. The lack of significant differences in the long-term follow-up is probably related to a too small number of patients in each group. Another factor that could explain the lack of differences is the longer duration of AF in the group treated by ablation.
In conclusion, hybrid therapy might be a reasonable therapy for patients with anti-arrhythmic drug induced atrial flutter. The left atrial size was the strongest predictor of post-ablation recurrence of atrial fibrillation. Careful patient selection for hybrid therapy of atrial fibrillation may provide long term stable sinus rhythm. However, in patients with severe atrial remodeling, rhythm control could not be maintained in prolonged period. Therefore, rate control without AAD might be a more reasonable approach for long term treatment in these patients.
Figures and Tables
Fig. 1
Comparision of 12 month event free survival curves between hybrid and electrical cardioversion group patients. AF, atrial fibrillation.

Fig. 2
Comparision of long term event free survival curves between hybrid and electrical cardioversion group patients. AF, atrial fibrillation.

ACKNOWLEDGEMENTS
This work was supported in part by Yonsei University Research Fund of 6-2008-0009 (JB Kim).
Notes
References
1. Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011. 123:e269–e367.
2. Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F Jr, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006. 354:934–941.

3. Knecht S, Hocini M, Wright M, Lellouche N, O'Neill MD, Matsuo S, et al. Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur Heart J. 2008. 29:2359–2366.

4. Stabile G, Bertaglia E, Turco P, Zoppo F, Iuliano A, Zerbo F, et al. Role of pulmonary veins isolation in persistent atrial fibrillation ablation: the pulmonary vein isolation in persistent atrial fibrillation (PIPA) study. Pacing Clin Electrophysiol. 2009. 32:Suppl 1. S116–S119.
5. Huang DT, Monahan KM, Zimetbaum P, Papageorgiou P, Epstein LM, Josephson ME. Hybrid pharmacologic and ablative therapy: a novel and effective approach for the management of atrial fibrillation. J Cardiovasc Electrophysiol. 1998. 9:462–469.

6. Schumacher B, Jung W, Lewalter T, Vahlhaus C, Wolpert C, Lüderitz B. Radiofrequency ablation of atrial flutter due to administration of class IC antiarrhythmic drugs for atrial fibrillation. Am J Cardiol. 1999. 83:710–713.

7. Reithmann C, Hoffmann E, Spitzlberger G, Dorwarth U, Gerth A, Remp T, et al. Catheter ablation of atrial flutter due to amiodarone therapy for paroxysmal atrial fibrillation. Eur Heart J. 2000. 21:565–572.

8. Turco P, De Simone A, La Rocca V, El Jamal B, Nocerino P, Astarita C, et al. Long-term results of hybrid therapy in patients with atrial fibrillation who develop atrial flutter during flecainide infusion. Pacing Clin Electrophysiol. 2005. 28:Suppl 1. S124–S127.

9. Stevenson WG, Sager PT, Friedman PL. Entrainment techniques for mapping atrial and ventricular tachycardias. J Cardiovasc Electrophysiol. 1995. 6:201–216.

10. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998. 339:659–666.

11. Lazar S, Dixit S, Marchlinski FE, Callans DJ, Gerstenfeld EP. Presence of left-to-right atrial frequency gradient in paroxysmal but not persistent atrial fibrillation in humans. Circulation. 2004. 110:3181–3186.

12. Murdock CJ, Kyles AE, Yeung-Lai-Wah JA, Qi A, Vorderbrugge S, Kerr CR. Atrial flutter in patients treated for atrial fibrillation with propafenone. Am J Cardiol. 1990. 66:755–757.

13. Tai CT, Chiang CE, Lee SH, Chen YJ, Yu WC, Feng AN, et al. Persistent atrial flutter in patients treated for atrial fibrillation with amiodarone and propafenone: electrophysiologic characteristics, radiofrequency catheter ablation, and risk prediction. J Cardiovasc Electrophysiol. 1999. 10:1180–1187.

14. Nabar A, Rodriguez LM, Timmermans C, Smeets JL, Wellens HJ. Radiofrequency ablation of "class IC atrial flutter" in patients with resistant atrial fibrillation. Am J Cardiol. 1999. 83:785–787.

15. Nabar A, Rodriguez LM, Timmermans C, van Mechelen R, Wellens HJ. Class IC antiarrhythmic drug induced atrial flutter: electrocardiographic and electrophysiological findings and their importance for long term outcome after right atrial isthmus ablation. Heart. 2001. 85:424–429.

16. Brooks AG, Stiles MK, Laborderie J, Lau DH, Kuklik P, Shipp NJ, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm. 2010. 7:835–846.

17. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005. 111:1100–1105.

18. Fetsch T, Bauer P, Engberding R, Koch HP, Lukl J, Meinertz T, et al. Prevention of atrial fibrillation after cardioversion: results of the PAFAC trial. Eur Heart J. 2004. 25:1385–1394.

19. Reithmann C, Dorwarth U, Dugas M, Hahnefeld A, Ramamurthy S, Remp T, et al. Risk factors for recurrence of atrial fibrillation in patients undergoing hybrid therapy for antiarrhythmic drug-induced atrial flutter. Eur Heart J. 2003. 24:1264–1272.

20. Stabile G, De Simone A, Turco P, La Rocca V, Nocerino P, Astarita C, et al. Response to flecainide infusion predicts long-term success of hybrid pharmacologic and ablation therapy in patients with atrial fibrillation. J Am Coll Cardiol. 2001. 37:1639–1644.

21. Wazni O, Marrouche NF, Martin DO, Gillinov AM, Saliba W, Saad E, et al. Randomized study comparing combined pulmonary vein-left atrial junction disconnection and cavotricuspid isthmus ablation versus pulmonary vein-left atrial junction disconnection alone in patients presenting with typical atrial flutter and atrial fibrillation. Circulation. 2003. 108:2479–2483.

22. Ortiz J, Niwano S, Abe H, Rudy Y, Johnson NJ, Waldo AL. Mapping the conversion of atrial flutter to atrial fibrillation and atrial fibrillation to atrial flutter. Insights into mechanisms. Circ Res. 1994. 74:882–894.

23. Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol. 2008. 51:779–786.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download