Abstract
Purpose
Many pediatric urologists still favor using prophylactic antibiotics to treat children with vesicoureteral reflux (VUR). However, breakthrough infection sometimes occurs, leading to significant increases in morbidity as a result of renal scarring. Therefore, we tested whether abnormal renal scan and other factors are predictive of breakthrough infection using univariate analyses.
Materials and Methods
We retrospectively reviewed the medical records of 163 consecutive children who were diagnosed with vesicoureteral reflux between November 1997 and June 2010. Clinical parameters for the statistical analysis included form of presentation, gender, age, VUR grade, laterality, presence of intrarenal reflux, class of antibiotic drug, and presence of abnormal renal scan by Dimercapto-succinic acid. Clinical parameters used for prognostic factors were established by univariate analyses. Fisher's exact test and unpaired t-test were done using SPSS software [SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA)].
Results
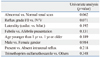
Breakthrough infection developed in 61 children (48.0%). A total of 58 children (45.7%) had abnormal renal scans. Time to development of breakthrough infection was significantly longer in girls (9.0±8.2 months) than in boys (5.8±4.8 months, p<0.05). On univariate analysis, though statistically not significant, the most predictive factor of breakthrough infection was abnormal renal scan (p=0.062). In patients with abnormal renal scans, breakthrough infection was not associated with mode of presentation, gender, grade or prophylactic antibiotics. However, there was a significant difference between patients younger than 1 year and those 1 year old or older. Mean±SD age at diagnosis of VUR in patients with breakthrough infection (1.14±3.14) was significantly younger than in those without breakthrough infection (5.05±3.31, p=0.009). There was also a significant difference between patients with bilateral or unilateral reflux (p=0.028).
Conclusion
Our data showed that abnormal renal scan was the most predictive factor of breakthrough infection and demonstrated statistical significance in patients under the age of 1 year. Parents and physicians should remain aware that these patients are at high risk of breakthrough urinary tract infection, which may potentially lead to renal damage.
Though two-thirds of children with urinary tract infections (UTIs) present no vesicoureteral reflux (VUR), vesicoureteral reflux still plays a role in the pathogenesis of urinary tract infection in children. The main therapeutic options available for primary reflux are administration of prophylactic antibiotics, endoscopic injection and antireflux surgery.1 The purpose of prophylactic administration of low dose antibiotics is to minimize bacterial growth in order to prevent pyelonephritis and renal scarring.2-4 However, breakthrough infection sometimes occurs, leading to significant increases in morbidity as a result of renal scarring. So, it is important to provide an estimate of the likelihood of breakthrough infection to allow for effective treatment of children with reflux. The American Urological Association panel on pediatric VUR guidelines revealed that the presence of renal scarring plays a major role in determining treatment options for children with VUR.1 If a renal scar is present, aggressive management is recommended to prevent further renal parenchymal damage, which can cause hypertension and end stage renal disease in some patients.5,6 Recently, Mingin, et al.7 reported that children with an abnormal renal scan are at an increased risk of breakthrough infection. Breakthrough infection is caused by a number of clinical factors. Using univariate analyses, we tested whether abnormal renal scan and other factors are predictive of breakthrough infection.
We retrospectively reviewed the medical records of 163 consecutive children who were diagnosed with vesicoureteral reflux. They were treated by two doctors (one pediatrician, one urologist) at our hospital between November 1997 and June 2010. Twenty and 11 patients were excluded due to the lack of Dimercapto-succinic acid (DMSA) scan data and follow-up loss during prophylactic antibiotic use, respectively. Five patients with neurogenic bladder and apparent voiding dysfunction were also excluded. A total of 127 children were enrolled in the statistical study. The primary outcome of a breakthrough infection was defined as the development of febrile UTI in patients receiving prophylactic antibiotics, and its diagnostic criteria included acute onset of high grade fever (38℃ or greater), pyuria (white blood cell count greater than 100 per high power field in urine sample sediment), positive urine bacterial culture, blood C-reactive protein of 4 mg/mL or greater, and white blood cell count of 10000/mL or greater. Clinical parameters for the statistical analysis included form of presentation, gender, age, VUR grade, laterality, presence of intrarenal reflux, class of antibiotic drug, and presence of abnormal renal scan by DMSA. Reflux degree on voiding cystourethrogram (VCUG) was graded according to the International Reflux Study classification.1 Abnormal renal scan performed basically at the time of acute febrile UTI was defined as positive upon presence of one or several parenchymal lesions and a small kidney of less than 40% on split renal function. The main antibiotic drug used for prophylaxis was trimethoprim-sulfamethoxazole and patient compliance was confirmed by questioning the parents every 1 or 2 months at outpatient follow-up. In patients receiving prophylactic antibiotics, repeat VCUG was performed annually until resolution. All boys in the study were not circumcised. Urine was bag collected if voiding was not independent. The majority of the children who had breakthrough infection underwent antireflux surgery. Clinical parameters used for prognostic factors were established by univariate analyses. Unpaired t-test was used to compare numerical data sets. A p-value <0.05 was considered significant. Fisher's exact test and unpaired t-test were conducted using SPSS software [SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA)].
Table 1 lists the patient demographics of the study. Breakthrough infection developed in 61 children (48.8%). Among the patients, 39 of 80 boys (48.8%) and 22 of 47 girls (46.8%) experienced breakthrough infection. There was not a significant difference between the genders. A total of 58 children (45.7%) had abnormal renal scans. Regarding the time to development of breakthrough infection, it was significantly longer in girls (9.0±8.2 months) than in boys (5.8±4.8 months, p<0.05). On univariate analysis patients with breakthrough infection were not at risk for age, gender, reflux laterality, presence of intrarenal reflux, choice of prophylactic antibiotics, or mode of presentation. However, the most predictive factor of breakthrough infection was abnormal renal scan (p=0.062). The second most predictive factor was higher reflux grade (p=0.071) (Table 2). In patients with abnormal renal scans breakthrough infection was not associated with mode of presentation, gender, grade or prophylactic antibiotics. However, there was significant difference between patients younger than 1 year and those 1 year old or older. Mean±SD age at diagnosis of VUR in patients with breakthrough infection (1.14±3.14) was significantly younger than in those without breakthrough infection (5.05±3.31, p=0.009). There was also a significant difference between patients with bilateral or unilateral reflux (p=0.028) (Table 3).
DMSA renal scan allows for cortical imaging and is currently used to evaluate children with urinary tract infections and vesicoureteral reflux.8 DMSA renal scan is able to highlight renal scars several months after resolution of acute infection. Agras, et al.9 proposed that DMSA scintigraphy be performed more than 6 months after pyelonephritis for the diagnosis of renal scarring. Sometimes, it is not easy to differentiate renal scars from an acute inflammatory lesion on DMSA renal scan, which are both seen in acute infections. Mingin, et al.7 reported that children with an abnormal renal scan detected by DMSA are at increased risk of breakthrough infection in patients with VUR. In our data, even though it was statistically not significant, abnormal renal scan was the most predictive factor of breakthrough infection. Moreover, in the patients under the age of 1 year, abnormal renal scan demonstrated statistical significance (p=0.001). This result supports the findings of Mingin, et al.7 and the concept of pediatric VUR guidelines, which revealed that the presence of renal scarring plays a major role in determining treatment options in children with VUR.1 The association between high reflux grade and breakthrough infection is also well documented in previous reports.10,11 Soylu, et al.11 reported that upon multivariate analysis, grade IV-V reflux (OR 12.4) was an independent indicator of renal scarring, a potential result of breakthrough infection. Our data also revealed higher reflux grade to be the second most predictive factor of breakthrough infection. However, the fact that abnormal renal scan rather than reflux grade was a more prominent risk factor for breakthrough infection in our study indicates that renal damage itself, more than reflux grade, is associated with the onset of breakthrough infection. This indicates that some unidentified host factors may be involved in susceptibility to and the extent of pyelonephritis. Previously, Kanematsu, et al.12 showed that secretor status, determined using the hemagglutination inhibition assay, is associated with abnormal renal scan, suggesting an unrecognized host disposition that affects the clinical course of primary VUR. Potentially, as published in other papers, bladder and bowel dysfunction may very well be one such factor, though we did not study this factor, because many of our patients were not yet toilet-trained.13 Further investigation into the mechanisms of recurrent UTIs and abnormal renal scans may be needed in the future. The mean age of the patients with an abnormal renal scan and breakthrough infection was significantly younger compared to those without infection in our results (p=0.01) (Table 3). This result is in accordance with the findings of Shiraishi who showed mean±SD age at diagnosis of VUR with breakthrough infection (1.92±3.06) was significantly younger than in those without breakthrough infection (3.83±3.44, p<0.01),14 further indicating that some host factors may be involved in the susceptibility to and extent of pyelonephritis.14 There was also significant difference between bilateral and unilateral reflux in patients with abnormal renal scan and breakthrough infection (Table 3). This is consistent with the findings of Park, et al.15 who showed bilateral VUR during the first year after birth significantly increased the risk of recurrent UTI. Our study has some bias of background of patients because some of our patients were referred to our center from primary physicians. Even though we performed DMSA renal scan basically at the time of acute pyelonephritis, some of the referred patients remained indeterminate in regards to whether abnormal renal scan was caused by acute inflammation or renal scarring. Our cases might have permanent cortical defect and transient defect caused by acute pyelonephritis to a variable extent. However, regardless of the causes of cortical defect, our result has shown that abnormal renal scan was the most predictive risk factor of breakthrough infection, especially in patients under the age of 1 year.16,17 Notwithstanding, we still need prospective data collected in a standardized fashion to be able to draw further conclusions. In conclusion, although the options to treat and follow children with abnormal renal scans vary for individuals, parents and physicians should remain aware that such patients, especially under the age of 1 year, are at high risk of breakthrough urinary tract infection, which may potentially lead to renal damage.
Figures and Tables
References
1. Elder JS, Peters CA, Arant BS Jr, Ewalt DH, Hawtrey CE, Hurwitz RS, et al. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol. 1997. 157:1846–1851.

2. Garin EH, Olavarria F, Garcia Nieto V, Valenciano B, Campos A, Young L. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics. 2006. 117:626–632.

3. Pennesi M, Travan L, Peratoner L, Bordugo A, Cattaneo A, Ronfani L, et al. Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics. 2008. 121:e1489–e1494.

4. Roussey-Kesler G, Gadjos V, Idres N, Horen B, Ichay L, Leclair MD, et al. Antibiotic prophylaxis for the prevention of recurrent urinary tract infection in children with low grade vesicoureteral reflux: results from a prospective randomized study. J Urol. 2008. 179:674–679.

5. Noe HN. The long-term results of prospective sibling reflux screening. J Urol. 1992. 148(5 Pt 2):1739–1742.

6. Walker RD. Renal functional changes associated with vesicoureteral reflux. Urol Clin North Am. 1990. 17:307–316.

7. Mingin GC, Nguyen HT, Baskin LS, Harlan S. Abnormal dimercapto-succinic acid scans predict an increased risk of breakthrough infection in children with vesicoureteral reflux. J Urol. 2004. 172:1075–1077.

8. Sweeney B, Cascio S, Velayudham M, Puri P. Reflux nephropathy in infancy: a comparison of infants presenting with and without urinary tract infection. J Urol. 2001. 166:648–650.

9. Agras K, Ortapamuk H, Naldöken S, Tuncel A, Atan A. Resolution of cortical lesions on serial renal scans in children with acute pyelonephritis. Pediatr Radiol. 2007. 37:153–158.

10. Caione P, Villa M, Capozza N, De Gennaro M, Rizzoni G. Predictive risk factors for chronic renal failure in primary high-grade vesico-ureteric reflux. BJU Int. 2004. 93:1309–1312.
11. Soylu A, Demir BK, Türkmen M, Bekem O, Saygi M, Cakmakçi H, et al. Predictors of renal scar in children with urinary infection and vesicoureteral reflux. Pediatr Nephrol. 2008. 23:2227–2232.

12. Kanematsu A, Yamamoto S, Yoshino K, Ishitoya S, Terai A, Sugita Y, et al. Renal scarring is associated with nonsecretion of blood type antigen in children with primary vesicoureteral reflux. J Urol. 2005. 174(4 Pt 2):1594–1597.

13. Peters CA, Skoog SJ, Arant BS Jr, Copp HL, Elder JS, Hudson RG, et al. Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. J Urol. 2010. 184:1134–1144.

14. Shiraishi K, Yoshino K, Watanabe M, Matsuyama H, Tanikaze S. Risk factors for breakthrough infection in children with primary vesicoureteral reflux. J Urol. 2010. 183:1527–1531.

15. Park S, Han JY, Kim KS. Risk factors for recurrent urinary tract infection in infants with vesicoureteral reflux during prophylactic treatment: effect of delayed contrast passage on voiding cystourethrogram. Urology. 2011. 78:170–173.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download