Abstract
Purpose
Neisseria meningitidis is a leading cause of bacterial meningitis in young adults. University students, especially those living in dormitories, have been known to be at increased risk of meningococcal disease. We performed a longitudinal study to determine the carriage rates of N. meningitidis and the changes thereof.
Materials and Methods
We recruited Inha University freshmen who were, at that time, admitted to a student dormitory. A pharyngeal swab was taken from all participant who were also asked to complete a questionnaire. This was repeated four weeks later.
Results
A total of 136 students were enrolled at the first culture. After four weeks, 128 students were enrolled, including 106 re-participants. The overall carriage rates changed from 11.8% to 14.1%. In analysis of the 106 re-participants, "visiting to pubs" was associated with carriage of N. meningitis for both the first (p=0.047) and second cultures (p=0.026). Serogroup C was found to be the most frequent serogroup (5 isolates), while 3 isolates were found from serogroup B. The most prevalent PorA types were P1.22,14-6 (4 isolates) and P1.19,15 (3 isolates). The DNA sequences of PorA VR2 were changed in 2 students during prolonged carriage.
Conclusion
The meningococcal carriage rate among first year university students who resided in a dormitory did not significantly increase over 4-week interval between cultures, which is markedly different from those reported in Western studies. Close social contact appeared to be related with carriage. Our data also revealed diversity in PorA types, suggesting the possibility of rapid mutation of the PorA gene during the 4-week interval.
Neisseria meningitidis is a leading cause of bacterial meningitis in young adults.1 It has been known that bringing together groups of young adults, such as military recruits, is associated with epidemic meningococcal disease. The congregation of students on university campuses, and in particular those living in university dormitories, may share some of the same characteristics of concern with meningococcal disease outbreaks among military recruits.
A study in Maryland conducted by Harrison, et al.2 indicated that the overall incidence of meningococcal disease among university students was comparable to that among the U.S. population of people of the same age (1.7/100000 and 1.4/100000, respectively); meanwhile, rates of disease among students living in dormitories were higher than those among students living off campus (3.2/100000 and 1.0/100000, respectively; p=0.05). In 2000, the Advisory Committee on Immunization Practices and the Committee on Infectious Diseases of the American Academy of Pediatrics concluded that college students, especially those living in dormitories, are at moderately increased risk for meningococcal disease, compared with other persons of the same age, and recommended meningococcal vaccination for college freshmen.3
Between 2001 and 2006, 7 to 38 probable cases of meningococcal disease were reported to the Korea Centers for Disease Control and Prevention annually. During the period from 2007 to 2008, the number of reported cases of meningococcal disease in Korea decreased (4 cases in 2007 and 1 cases in 2008). The disease has been regarded as a rare infection in Korea. Although carriage studies are important to improve our understanding of the epidemiology and pathogenesis of meningococcal disease, there have been no studies published on the epidemiology of meningococcal carriage or acquisition among university students in Korea, probably because of the low incidence of the disease. Therefore, we performed a longitudinal study of first year university students who resided in a student dormitory at the start of the academic year to determine the rates of carriage of N. meningitidis and thes changes thereof after one month, together with risk factors and serogroups.
We recruited Inha University freshmen who were, at that time, admitted to a university dormitory in the first week of March 2009. Each student was given an information sheet and a consent form. Those agreeing to take part in the study completed a questionnaire covering: personal characteristics, place of residence, recent symptoms of upper respiratory tract infection, recent history of antibiotics use, travel abroad, active smoking, intimate kissing in the past month and visits to nightclubs or pubs in the past two weeks. After each student had completed the questionnaire, a trained operator took a posterior pharyngeal swab behind the uvula.
A second round of swabbing was undertaken 4 weeks later. At the time of reswabbing, the questionnaire was repeated.
Cotton swabs were used and they were directly plated onto Thayer-Martin media. Strains were confirmed to be N. meningitidis by conventional methods including colony morphology, Gram staining, oxidase tests, catalase tests, carbohydrate fermentation tests, and the API NH kit.
Serogroups could not be determined by the slide agglutination method with polyclonal antisera (Difco, Detroit, MI, USA) because frequent non-specific reactions or cross reactions were observed. Therefore, serogrouping by polymerase chain reaction (PCR) was performed. For the detection and identification of serogroups A, B, C, Y, W135, a multiplex PCR was performed simultaneously with oligonucleotides in the siaD gene (serogroups B, C, Y, and W135) and in a gene cassette of orf-2 required for the biosynthesis of the capsule of serogroup A.4 For the detection and identification of serogroups 29E, X, and Z, the ctrA gene, exclusive to meningococci and forming part of the capsule biosynthesis locus, was chosen as the PCR target according to the methods described by Bennett, et al.5
Amplification of the porA gene by PCR and sequencing of the porA variable regions (VR1 and VR2) were performed as described by Sacchi, et al.6 All nucleotide sequences for each VR were aligned with reference to both the nucleotide and the corresponding amino acid sequences through a Web-accessible PorA database (http://neisseria.org/nm/typing/pora) and defined subtype families.
Data collected at the time of the pharyngeal swabs were used for the correlation analysis on carriage of N. meningitidis. Statistical analysis was conducted for crosstabs in Pearson Chi-Square statistics using the Statistical Package of Social Science software (SPSS, SPSS Inc., Chicago, IL, USA). Two-sided probability values of <0.05 were considered statistically significant. We did not perform multiple comparisons.
A total of 136 first year students were enrolled at the first culture. After four weeks, 128 students were enrolled at the second culture, including 106 re-participants. The overall carriage rates were 11.8% (16/136) at the first culture and 14.1% (18/128) at the second culture (Table 1). Among the follow-up results for the 106 re-participants who were reswabbed one month later, carriage rates did not change from the first culture to the second culture at 15.1% (16/106). Of the carriers initially identified, 75% (12/16) were still positive at the second culture. Of the 90 noncarriers who were followed up, 4.4% (4/90) acquired carriage and 95.6% (86/90) remained as noncarriers one month later.
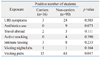
Table 2 shows the correlation analysis on carriage of N. meningitidis at the first culture of the 106 students who were followed up. Active smoking, visiting nightclubs, intimate kissing, travel abroad, and recent symptoms of upper respiratory tract infection were not significantly associated with being a carrier. Only "visiting pubs" two weeks before examinations was associated with carriage of N. meningitidis at both the first (p=0.047) and second cultures (p=0.026) in an analysis of the 106 students who were followed up. We also noted that there was no carriage at the first culture among 12 students who had a history of antibiotics use.
Table 3 summarizes the serogroup distribution among the meningococcal isolates in the 106 students who were followed up. Of the 16 total meningococcal isolates of the first culture, serogroup C was found to be the most frequent (5 isolates) and 3 isolates were found from serogroup B. We could not determine a serogroup by PCR for 8 isolates (non-groupable, 50%). Four students who were carriers at the first culture were discovered to be negative at the second culture. Two of them were non-groupable, one was from serogroup C and the other was from serogroup B. Of 90 noncarriers followed up, 4 students acquired carriage one month later at the second culture. Two isolates were from serogroup B, one was from serogroup 29E, and the other was from serogroup W135.
We obtained 18 meningococcal isolates out of 128 students who were enrolled at the second culture and 13 different PorA VR sequences were identified among them (Table 4). The most prevalent PorA types were P1.22,14-6 (4 isolates) and P1.19,15 (3 isolates). PorA types among the serogroup C isolates were relatively uniform, and serogroup C isolates expressing P1.22,14-6 was predominant, accounting for 66.6% (4/6) of all serogroup C isolates. We also analyzed and compared the PorA VR sequences of the 12 students who maintained their carrier state over the 4-week interval between cultures to assess the stability of the porA gene on prolonged carriage. PorA VR2 sequences were changed in 2 students, although their serogroups remained unchanged. One was serogroup B with a change in PorA type from P1.19-5,15-48 to P1.19-5,14-6 and the other was non-groupable with a change in PorA type from P.1.18-21,25-39 to P1.18-21,25-40.
Asymptomatic pharyngeal colonization of N. meningitidis in young adults is relatively common and an age-dependent phenomenon, with point prevalence carriage rates usually ranging from 10% to 35%.7,8 Neal, et al.9 performed a longitudinal study and reported that carriage rates of meningococci among university students increases rapidly throughout the first week of the term from 6.9% on day 1 to 23.1% on day 4. The average carriage rate during the first week of the term was 13.9%, with further increases throughout the term to 31.0% after one month. They also identified active smoking, intimate kissing and visiting nightclubs as risk factors for acquisition.
To gain information concerning the pharyngeal carriage of N. meningitidis among freshmen aged 20-22 years in Korea, those admitted to Inha University dormitory were enrolled in our study. Each student was swabbed twice at an interval of four weeks between cultures. We maintained the same swabbing technique using the same trained operators and culture conditions throughout the study to minimize the influence of confounding factors. In our results, the overall carriage rate was 11.8% (16/136) at the first culture, which is comparable with those reported in Western countries. However, the overall carriage rate did not significantly increase from 11.8% to 14.1% after 4 weeks. A few explanations may explain this result. In fact, the prevalence of known risk factors for acquisition among the students was lower compared to Western studies. The prevalence was 4.41% (6/136) for active smoking, 10.29% (14/136) for intimate kissing and 1.47% (2/136) for visiting nightclubs at the first culture in our study. In comparison, in a study for social behavior and meningococcal carriage among British teenagers,10 the corresponding prevalence was 21.48% (2936/13668), 44.77% (6133/13697) and 62.06% (8447/13611), respectively. Lower prevalence of risk factors for acquisition in our study could explain the insignificant change of carriage rates over 4 weeks, to a certain extent. Furthermore, it might have been influenced by the difference in social behavior patterns of Korean students, which is more reserved, less communicative, resulting in decreased social mixing. Therefore, different results may be shown if we examined the students for longer periods of time.
A number of risk factors have been shown to be associated with meningococcal carriage. There are slightly fewer carriers in females than in males. Those with viral or bacterial respiratory tract infections could be at high risk to become carriers.11 Smoking, whether active or passive, is one of the strongest risk factors for becoming a meningococcal carrier12-14 and low socioeconomic position in some populations appears to raise the risk of carriage.15 These risk factors are known to be independently correlated with carriage. Also, concerning the effect of bringing together groups of young adults, there was a study which identified a higher carriage rate in catered halls than in self-catered halls.9 On the other hand, it was reported that there was no evidence that increasing levels of crowding was associated with increasing levels of meningococcal carriage.10 The Inha University dormitory where our study was performed is a catered hall of residence with around 1000 students, and four students usually share a room which has an area of 21.49 m2. Interestingly, active smoking, visiting nightclubs, intimate kissing, travel abroad, and recent symptoms of upper respiratory tract infection were not significantly associated with being a carrier at our first culture. This might be because the number of carriers in our study was too small to draw a meaningful interpretation. Indeed, students who smoked, kissed or visited nightclubs were small in number as shown in Table 2. It might also reflect cultural characteristics in Korea, which are less permissive. Nonetheless, visiting pubs was correlated with meningococcal carriage at both the first (p=0.047) and second cultures (p=0.026), which was similar to other studies. We also noted that there was no carriage at the first culture among 12 students who had a history of antibiotics use. This was probably because antibiotics inhibited the growth of the bacteria.
N. meningitidis is divided into 13 serogroups based on the immunological specificity of the capsular polysaccharide. It has been known that patient strains are encapsulated and five of these serogroups (A, B, C, W135 and Y) cause more than 90% of the invasive disease worldwide.16 In contrast, 50% of the strains isolated from carriers lack a capsule and are therefore serologically not serogroupable.17,18 In our study, serogroups could not be determined by the slide agglutination method with polyclonal antisera because frequent non-specific reactions or cross reactions were frequently observed. This was probably due to the lack of a capsule in the strains isolated from carriers or there could have been problems related to culturing the isolates. Claus, et al.19 commented that there are two essential reasons for the lack of capsule - the deletion of the capsule locus or down-regulations of the expression of the capsule, either temporarily or permanently, by one of a number of genetic mechanisms. Until recently, nongroupable isolates were thought to be nonpathogenic. On the contrary, it has become progressively clear that capsular expression is phase variable,20 and the loss of capsule may enhance the organism's ability to colonize the nasopharynx.
Caugant, et al.21 performed a comparative study of the expressed capsular antigens in a collection of 667 meningococci isolated from patients and carriers in three European countries to better understand the relationship between carrier and disease-causing strains. Serogroup B strains was predominant among both patient and carrier isolates. Although marked differences between countries were observed in the serogroup distribution of patient isolates, the serogroup distribution of the carrier isolates was similar between countries, with predominance of serogroup B among isolates expressing a capsular polysaccharide, followed by serogroup Y. In our study, serogrouping by PCR was performed for the detection and identification of serogroups A, B, C, Y, W135, X, Z and 29E. Of 16 total meningococcal isolates of the first culture, serogroup C was found to be the most frequent (31.3%) and serogroup B followed (18.8%). We could not determine a serogroup by PCR for 8 isolates (genetically non-groupable, 50%).
The currently available meningococcal vaccines are based on the capsular polysaccharides of meningococcal serogroups A, C, W-135 and Y. None of these vaccines provide protection against serogroup B meningococci, which is the prevalent serogroup in Europe, North America, South America, and Australia, because the capsular polysaccharide of meningococcal serogroup B is unable to stimulate a protective immune response.22 Other alternatives for meningococcal outer membrane protein (OMP)-based vaccines have been investigated to prevent meningococcal disease, regardless of their serogroup.23 The PorA OMP, characterized by genosubtyping of the VR1 and VR2 of the porA gene, is highly immunogenic and is considered to be an important component in protein-based vaccines against meningococcal disease. However, PorA shows a high degree of antigenic variation which is used for serologic differentiation of isolates.24 Consequently, newer PorA-based vaccines have to consider the prevalence of genosubtypes and contain multiple antigenic variants of PorA.25 Our data revealed the diversity of the PorA types of N. meningitidis, although the number of isolates analyzed was small. Moreover, PorA VR2 sequences were changed in 2 students during prolonged carriage in our study, which is different from the results of a group of military recruits in the UK.26 This suggests the possibility of either rapid mutation of the porA gene or horizontal exchange of genetic material between strains during the 4-week interval between cultures. It also reveals the difficulty in designing a useful OMP vaccine.
This study was the first report of rates of carriage and acquisition of N. meningitidis among freshmen in a university dormitory in Korea, together with serogroups and genosubtypes. The carriage rate is comparable with those reported from other studies. However, it did not significantly increase after four weeks, which is markedly different from previous studies in Western countries. This suggests that freshmen at this university may be at low risk of clusters of cases with meningococcal disease, and may therefore be at a low risk of outbreaks. Inversely, this low rate of carriage acquisition after one month might explain the low incidence of meningococcal disease in freshmen in Korea. As we mentioned, transmission dynamics in freshmen in Korea may differ from those in western countries. Therefore, it will be helpful to continue follow-up swabbing in the same dormitory or to perform a longitudinal study at other universities on a large scale, to assess how it compares with Western studies.
Figures and Tables
Table 2
Correlation Analysis on Carriage of Neisseria meningitidis at the First Culture of the 106 Students Who Were Followed Up (Pearson Chi-Square)
References
1. Rosenstein NE, Perkins BA, Stephens DS, Lefkowitz L, Cartter ML, Danila R, et al. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J Infect Dis. 1999. 180:1894–1901.

2. Harrison LH, Dwyer DM, Maples CT, Billmann L. Risk of meningococcal infection in college students. JAMA. 1999. 281:1906–1910.

3. Meningococcal disease and college students. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000. 49:13–20.
4. Taha MK. Simultaneous approach for nonculture PCR-based identification and serogroup prediction of Neisseria meningitidis. J Clin Microbiol. 2000. 38:855–857.

5. Bennett DE, Mulhall RM, Cafferkey MT. PCR-based assay for detection of Neisseria meningitidis capsular serogroups 29E, X, and Z. J Clin Microbiol. 2004. 42:1764–1765.

6. Sacchi CT, Whitney AM, Popovic T, Beall DS, Reeves MW, Plikaytis BD, et al. Diversity and prevalence of PorA types in Neisseria meningitidis serogroup B in the United States, 1992-1998. J Infect Dis. 2000. 182:1169–1176.

7. Caugant DA, Høiby EA, Magnus P, Scheel O, Hoel T, Bjune G, et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiol. 1994. 32:323–330.

8. Claus H, Maiden MC, Wilson DJ, McCarthy ND, Jolley KA, Urwin R, et al. Genetic analysis of meningococci carried by children and young adults. J Infect Dis. 2005. 191:1263–1271.

9. Neal KR, Nguyen-Van-Tam JS, Jeffrey N, Slack RC, Madeley RJ, Ait-Tahar K, et al. Changing carriage rate of Neisseria meningitidis among university students during the first week of term: cross sectional study. BMJ. 2000. 320:846–849.

10. MacLennan J, Kafatos G, Neal K, Andrews N, Cameron JC, Roberts R, et al. Social behavior and meningococcal carriage in British teenagers. Emerg Infect Dis. 2006. 12:950–957.

11. Stephens DS. Uncloaking the meningococcus: dynamics of carriage and disease. Lancet. 1999. 353:941–942.

12. Stuart JM, Cartwright KA, Robinson PM, Noah ND. Effect of smoking on meningococcal carriage. Lancet. 1989. 2:723–725.

13. Kremastinou J, Blackwell C, Tzanakaki G, Kallergi C, Elton R, Weir D. Parental smoking and carriage of Neisseria meningitidis among Greek schoolchildren. Scand J Infect Dis. 1994. 26:719–723.

14. Blackwell CC, Tzanakaki G, Kremastinou J, Weir DM, Vakalis N, Elton RA, et al. Factors affecting carriage of Neisseria meningitidis among Greek military recruits. Epidemiol Infect. 1992. 108:441–448.

15. Davies AL, O'Flanagan D, Salmon RL, Coleman TJ. Risk factors for Neisseria meningitidis carriage in a school during a community outbreak of meningococcal infection. Epidemiol Infect. 1996. 117:259–266.

16. Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J. 2004. 23:12 Suppl. S274–S279.

17. Caugant DA, Kristiansen BE, Frøholm LO, Bøvre K, Selander RK. Clonal diversity of Neisseria meningitidis from a population of asymptomatic carriers. Infect Immun. 1988. 56:2060–2068.

18. Ala'Aldeen DA, Neal KR, Ait-Tahar K, Nguyen-Van-Tam JS, English A, Falla TJ, et al. Dynamics of meningococcal long-term carriage among university students and their implications for mass vaccination. J Clin Microbiol. 2000. 38:2311–2316.
19. Claus H, Maiden MC, Maag R, Frosch M, Vogel U. Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology. 2002. 148(Pt 6):1813–1819.

20. Swartley JS, Marfin AA, Edupuganti S, Liu LJ, Cieslak P, Perkins B, et al. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci U S A. 1997. 94:271–276.
21. Caugant DA, Tzanakaki G, Kriz P. Lessons from meningococcal carriage studies. FEMS Microbiol Rev. 2007. 31:52–63.

22. Finne J, Leinonen M, Mäkelä PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983. 2:355–357.
23. Herbert MA, Heath PT, Mayon-White RT. Meningococcal vaccines for the United Kingdom. Commun Dis Rep CDR Rev. 1995. 5:R130–R135.
24. Feavers IM, Fox AJ, Gray S, Jones DM, Maiden MC. Antigenic diversity of meningococcal outer membrane protein PorA has implications for epidemiological analysis and vaccine design. Clin Diagn Lab Immunol. 1996. 3:444–450.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download