Abstract
Purpose
This study aimed to elucidate whether stone removal by extracorporeal shock wave lithotripsy (ESWL) is associated with delayed chronic kidney disease (CKD) progression.
Materials and Methods
We conducted a retrospective analysis of 131 nephrolithiasis patients with stage 3 and 4 CKD. We collected baseline clinical and laboratory data, kidney stone characteristics, and history of receiving ESWL. We classified study patients into two groups according to whether they underwent ESWL or not (Non-ESWL group vs. ESWL group). We initially compared annual estimated glomerular filtration rate (eGFR) changes of Non-ESWL group with those of ESWL group before undergoing ESWL. In the next step, we sought to compare annual eGFR changes in the same patients before and after ESWL. Finally, we compared annual eGFR changes between success and failure groups among patients undergoing ESWL.
Results
The mean age of the patients was 62 years and 72.5% were male. The mean observation period was 3.2 years. Non-ESWL group and ESWL group before undergoing ESWL showed similar annual eGFR changes (-1.75±6.5 vs. -1.63±7.2 mL/min/1.73 m2/year, p=0.425). However, eGFR declined slower after undergoing ESWL than before ESWL (annual eGFR changes, -0.29±6.1 vs. -1.63±7.2 mL/min/1.73 m2/year, p<0.05). In addition, among patients in ESWL group, eGFR declined faster in the failure group than in the success group (annual eGFR change, -1.01±4.7 vs. -0.05±5.2 mL/min/1.73 m2/year, p<0.05).
Kidney stone or nephrolithiasis commonly affects 1.7 to 14.8% of the general population, and both prevalence and incidence have increased globally across age, gender, and race, resulting in increased morbidity.1 In cases of kidney stones arising from rare hereditary disorders or staghorn stones resulting from chronic urinary tract infection with urease-containing bacteria, kidney stones have evidently been reported to cause significant chronic renal damage and sometimes lead to end stage renal disease (ESRD).2-5 However, the United States Renal Data System 2010 Annual Data Report showed that only 2.4% of all ESRD were caused by these kinds of kidney stones.6
Although the impact of kidney stones on the renal function has not yet been clearly elucidated, recent studies showed that kidney stones might be associated with increased risk of development of chronic kidney disease (CKD).7-10 However, in patients who already have CKD, little is known as for whether the presence of kidney stones influences the progression of CKD and whether removal of kidney stones could modify the course of CKD progression. Moreover, long-term effect of extracorporeal shock wave lithotripsy (ESWL) on the renal function deterioration in CKD population has not been studied to date. Therefore, we undertook this study to elucidate whether stone removal by ESWL would be associated with delayed CKD progression in CKD patients with kidney stones that are not associated with urinary tract obstruction.
This is a retrospective single center study approved by institutional review board. The study subjects initially consisted of patients with nephrolithiasis, who were identified by using International Classification of Diseases, Tenth Revision (ICD-10) codes: N20.0 (calculus of kidney), N20.9 (urinary calculus, unspecified), and N23 (unspecified renal colic). A comprehensive review of medical records was then conducted to confirm, or reject potential cases of nephrolithiasis without hydronephrosis. Between January 2002 and December 2009, a total of 2022 patients were confirmed by ultrasonography and/or computed tomography to have kidney stones without hydronephrosis. Of these patients, 169 were eligible for the study because they had an estimated glomerular filtration rate (eGFR) between 15 and 60 mL/min/1.73 m2 before the initial diagnosis of nephrolithiasis, which were calculated using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation: eGFR=186×[serum creatinine (mg/dL)]-1.154×[age]-0.203×[0.742 if female]×[1.210 if black].11 eGFR had to be documented consecutively at least twice before the diagnosis of nephrolithiasis to confirm that patients had CKD. It also had to be followed at least once every 6 months after the diagnosis to include patients in this study. Fifteen patients were excluded due to inadequate information on eGFR. Patients who had transplanted kidney or solitary kidney were excluded (n=8). Patients with staghorn stone were also excluded (n=3) because those stones resulted in complete obstruction of renal pelvis. Patients who had been followed for less than 12 months were excluded (n=10). We also excluded patients with polycystic kidney disease (n=1) and/or genitourinary system cancer (n=1). Consequently, we identified 131 patients who met the study criteria and these patients were included in the final analyses (Fig. 1).
Using a retrospective review of patient medical records, the following demographic and clinical data were collected: age at the time of diagnosis; gender; height; weight; comorbidities including diabetes mellitus, hypertension, and cardiovascular disease; stone characteristics including location (unilateral vs. bilateral), number (solitary vs. multiple), and diameter; and history of receiving ESWL. We also collected laboratory parameters including hemoglobin, calcium, phosphorus, blood urea nitrogen, serum creatinine, total protein, albumin, and aspartate/alanine aminotransferase. Proteinuria was defined when random urine protein-to-creatinine ratio was greater than 0.2 mg/mg.
We classified study patients into two groups according to whether they underwent ESWL or not (Non-ESWL group vs. ESWL group). Annual eGFR change was calculated using all available data during follow-up period for Non-ESWL patients. In contrast, pre-ESWL and post-ESWL annual eGFR changes were respectively estimated for study patients who underwent ESWL. We used eGFR data up to 2 years before undergoing ESWL and additional consecutive eGFR data after ESWL for these patients. Annual eGFR change was estimated by using a linear regression model of time on eGFR in each patient, and it was defined as coefficient of regression of this regression model.
In terms of data related to ESWL, following variables were collected: sessions per stone, voltage, number of shocks per stone, and stone clearance rate at 3 months after ESWL. Success of stone clearance was defined as achieving stone-free status or having clinically insignificant residual fragments. On the contrary, failure of stone clearance was defined as showing no response or having significant residual fragments.
We initially compared annual eGFR changes of Non-ESWL group to those of ESWL group before undergoing ESWL (pre-ESWL). In the next step, we sought to compare annual eGFR changes during pre-ESWL period with those after undergoing ESWL (post-ESWL). Finally, we analyzed differences between success and failure groups among patients undergoing ESWL.
ESWL had been performed with EDAP LT-02 (Technomed, Lyon, France) until April 2005, and it was replaced by EDAP Sonolith Praktis since May 2005. ESWL therapy usually started at a low voltage of 5 kV until the patient became accustomed to the shocks, and the voltage was then gradually increased to a maximum of 9 kV. The average number of shocks per session was 2000-4000.
Continuous data are expressed as mean±standard deviation. Categorical data are presented as absolute values and percentages. Clinical characteristics of the Non-ESWL and pre-ESWL groups were compared with the use of Student t-test for continuous variables and the chi-square test or Fisher's exact test for categorical variables. Annual eGFR changes before and after ESWL in patients who underwent ESWL were compared using paired t-test. Owing to paucity of number of patients undergoing ESWL, characteristics between success and failure groups among patients receiving ESWL were compared with the use of Mann-Whitney U test for continuous variables and Fisher's exact test for categorical variables.
A p-value of less than 0.05 was considered to indicate statistical significance. All calculations were computed with the aid of PASW Statistics software (version 18).
The mean age of the study sample was 62 years (range, 28-82 years), and 72.5% were male. Of the 131 patients, 123 (93.9%) had CKD stage 3 at baseline. Primary renal diseases were diabetic nephropathy (40.5%), hypertensive nephropathy (32.1%), chronic glomerulonephritis (11.5%), and CKD from other causes (16.1%). Average systolic and diastolic blood pressures at baseline were 133.9 and 77.5 mm Hg, respectively. Mean diameter of kidney stones was 2.1±0.6 cm, and 48.1% of patients had bilateral kidney stones. Among all patients, 34 (26.0%) patients underwent ESWL for stone removal and received 3425±560 shocks per stone with mean voltage of 5.7±1.3 kV over 2.2±1.4 sessions per stone.
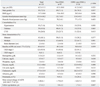
Table 1 shows clinical and laboratory findings between the Non-ESWL and Pre-ESWL groups. No differences in age, gender, body mass index (BMI), systolic and diastolic pressure, prevalence of comorbidities including hypertension, diabetes and CVD, stone characteristics, and baseline eGFR were found between the two groups. In addition, laboratory findings such as hemoglobin, calcium, phosphate, uric acid, cholesterol, and proteinuria were not different between the groups. Moreover, Non-ESWL group and pre-ESWL group showed similar annual eGFR changes (-1.75±6.5 vs. -1.63±7.2 mL/min/1.73 m2/year, p=0.425). However, follow-up duration of Non-ESWL group was longer than that of pre-ESWL group (3.0±1.2 vs. 1.7±1.1 years, p=0.047). However, total follow-up duration including both before and after ESWL was comparable to that of Non-ESWL group (3.0±1.2 vs. 3.9±1.2 years, p=0.152).
Patients who underwent ESWL were followed for 1.7 years before ESWL and for 2.3 years after ESWL. To clarify whether ESWL affected the renal function decline rate in CKD patients, annual eGFR changes before and after ESWL were compared. The result showed that eGFR after ESWL declined slower than eGFR before undergoing ESWL (annual eGFR changes, -0.29±6.1 vs. -1.63±7.2 mL/min/1.73 m2/year, p<0.05), as depicted in Fig. 2.
In terms of complications that occurred after ESWL, subcapsular hematoma occurred in 2.9% of patients, which was clinically insignificant. Although urinary tract infections also occurred in 8.7% of patients after ESWL, all cases were successfully cured with antibiotics therapy. Clinically significant urinary tract obstruction from stone fragments after ESWL occurred in 5.8% of patients, and ureteroscopic procedures were needed to decompress the obstruction for these patients.
Among patients undergoing ESWL, the success rate of stone clearance at 3 months after ESWL was 73.5%. To elucidate whether the stone clearance after ESWL is associated with annual eGFR changes after ESWL, we compared characteristics of patients between success and failure groups. we compared characteristics of patients between success and failure groups (Table 2). There were no differences in age, gender, BMI, blood pressure, prevalence of comorbidities, and stone characteristics between the groups. In addition, laboratory findings including hemoglobin, calcium phosphate, cholesterol, and proteinuria were comparable between the groups. Although baseline eGFR at the time of ESWL were slightly greater in the success group than in the failure group, the difference was not statistically significant. However, eGFR declined faster in the failure group than in the success group (annual eGFR change, -1.01±4.7 vs. -0.05±5.2 mL/min/1.73 m2/year, p<0.05).
In this retrospective study on 131 CKD patients with nephrolithiasis from a single center, we found that eGFR declined slower in patients who underwent ESWL than in patients who did not receive ESWL. In addition, we also found that eGFR declined faster after undergoing ESWL in the failure group than in the success group. Taken together, this study suggests that stone removal by ESWL is associated with delayed deterioration of renal function in CKD patients with nephrolithiasis, implying kidney stones per se might be associated with worsening kidney function in CKD patients.
In CKD patients, the rate of GFR decline is generally, known to be influenced by several non-modifiable risk factors such as type of kidney disease, race, baseline GFR, gender, and age.11 Besides these non-modifiable risk factors, a few modifiable patient characteristics such as proteinuria, serum albumin concentration, and blood pressure are known to be potent predictors of CKD progression.11 However, it is totally unexplored to date whether the presence of kidney stone modifies renal function decline rate in patients who already have CKD although many studies reported that kidney stones are associated with CKD, development.7-10 To answer this question, we sought to compare annual eGFR changes in the same patient before and after stone removal. In cases of accompanying hydronephrosis, patients' intrinsic GFR before stone removal may be obscured by coexisting obstructive uropathy, and the effect of kidney stones per se on GFR decline rate may not properly be evaluated in these patients. Therefore, we excluded nephrolithiasis patients with hydronephrosis or staghorn stones.
ESWL is one of the most widely used non-invasive treatment modality for uncomplicated small stones (<2.5 cm) in the kidney and upper urinary tract.12 However, it is currently unknown whether stone removal by ESWL improves long-term renal outcomes in CKD patients. Therefore, we traced eGFR changes before and after stone removal, especially among patients who underwent ESWL. Several earlier experimental animal studies argued against the efficacy of ESWL because it induces renal parenchymal injury, promoting further kidney function deterioration.13 Specifically, it induces dramatic vascular insult in which capillaries, veins and medium-to-small-diameter arteries are torn, accompanied by focal sites of parenchymal and subcapsular hemorrhage.14 Tubules also show features similar to the tears seen in the vessel walls. In addition, ESWL-treated kidneys show a short-term reduction in renal plasma flow.15 Moreover, ESWL-induced trauma triggers the infiltration of inflammatory cells at the site of the lesion.16 Taken together, ESWL trauma is regarded to cause scar formation and loss of functional renal mass.14 In contrast, however, other recent studies reported that renal function impairment associated with ESWL is largely resolved within 1 to 3 months and ESWL per se has no significant long-term effects on renal function.17,18 However, the patients included in these studies had normal renal function, and the effect of ESWL in CKD patients was not addressed. Interestingly, our present study showed that eGFR in ESWL group declined slower after stone removal than before undergoing ESWL, although annual eGFR changes before ESWL were comparable to those of nephrolithiasis patients in the Non-ESWL group. In addition, eGFR declined faster after undergoing ESWL in the failure group than in the success group. These results suggest that stone removal by ESWL is associated with delayed CKD progression in these patients. The mechanism behind this finding is unclear, but removal of kidney stones may improve urine flow and decrease repeated short-lived bouts of subclinical partial obstructive nephropathy caused by nephrolithiasis. Our findings also provide evidence that nephrolithiasis might be a risk factor for CKD progression because stone removal attenuated kidney function deterioration.
One might argue that acute renal parenchymal injury following ESWL was underestimated in this study. Due to the retrospective nature of our study and small number of patients who underwent ESWL, we could not assess the short-term effect of ESWL on CKD patients. However, there is a possibility that acute kidney injury caused by ESWL is largely reversible without leaving long-term adverse effects, unless major complications occur. While previous studies showed short-term decline of renal function after ESWL, no single study revealed long-term decline of renal function in patients without major ESWL complications. Moreover, it is unclear whether harmful effects of ESWL on renal function would outweigh positive effects from stone clearance by ESWL in the long term. In our study, most patients who underwent ESWL did not experience major ESWL complications. Although hematoma occurred in 2.9% of patients who received ESWL, it disappeared in time. In addition, although clinically significant urinary tract obstruction from stone fragments followed ESWL in 5.8% of patients, it was eventually resolved after ureteroscopic procedures. Moreover, even urinary tract infections that occurred in 8.7% of patients after ESWL were all successfully cured with antibiotics therapy. Therefore, it can be surmised that short-term harmful effects of ESWL waned over time, while long-term positive effect from stone clearance persisted. However, this hypothesis needs further investigation.
This study has several limitations. First, as a retrospective single-center study, it is subject to the biases inherent to this study design. Second, eGFR was used instead of measured GFR. Currently, measured GFR using iothalamate is considered to be the most accurate GFR. However, it is not usually used in most of institutes because it is cumbersome and inconvenient, and information on iothalamate-GFR was not available. Since previous studies revealed that creatinine-based eGFR correlates well with iothalamate-GFR, we used MDRD equation to calculate eGFR. Third, exact information about kidney stone components was not available because many stones were not analyzed and few patients underwent 24-hour urine chemistry tests. However, meticulous review of the radiologic examinations of stones revealed that no radiolucent stones were included in this study. Therefore, most patients in our study likely had calcium-based stones.
In conclusion, our study shows that stone removal by ESWL is associated with delayed deterioration of renal function in CKD patients with nephrolithiasis. Therefore, our findings suggest that ESWL for stone removal should be encouraged to prevent further worsening of kidney function in these patients. A prospective well-designed study with larger study sample is warranted to confirm our findings.
Figures and Tables
Fig. 1
Flow diagram indicating patient recruitment and exclusion. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; CT, computed tomography; US, ultrasonography.

Fig. 2
Comparison of annual eGFR decline between ESWL and Non-ESWL group. ESWL, extracorporeal shock wave lithotripsy; eGFR, estimated glomerular filtration rate.

References
1. Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010. 12:e86–e96.
2. Frymoyer PA, Scheinman SJ, Dunham PB, Jones DB, Hueber P, Schroeder ET. X-linked recessive nephrolithiasis with renal failure. N Engl J Med. 1991. 325:681–686.

3. Holmgren K, Danielson BG, Fellström B. Infection-induced urinary calculi and renal failure. Scand J Urol Nephrol. 1987. 21:219–223.

4. Assimos DG, Leslie SW, Ng C, Streem SB, Hart LJ. The impact of cystinuria on renal function. J Urol. 2002. 168:27–30.

5. Singh M, Chapman R, Tresidder GC, Blandy J. The fate of the unoperated staghorn calculus. Br J Urol. 1973. 45:581–585.

6. U S Renal Data System. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. USRDS 2010 Annual Data Report. 2010. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
7. Gillen DL, Worcester EM, Coe FL. Decreased renal function among adults with a history of nephrolithiasis: a study of NHANES III. Kidney Int. 2005. 67:685–690.

8. Rule AD, Bergstralh EJ, Melton LJ 3rd, Li X, Weaver AL, Lieske JC. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009. 4:804–811.

9. Vupputuri S, Soucie JM, McClellan W, Sandler DP. History of kidney stones as a possible risk factor for chronic kidney disease. Ann Epidemiol. 2004. 14:222–228.

10. Saucier NA, Sinha MK, Liang KV, Krambeck AE, Weaver AL, Bergstralh EJ, et al. Risk factors for CKD in persons with kidney stones: a case-control study in Olmsted County, Minnesota. Am J Kidney Dis. 2010. 55:61–68.

11. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002. 39:2 Suppl 1. S1–S266.
12. LeRoy AJ. Diagnosis and treatment of nephrolithiasis: current perspectives. AJR Am J Roentgenol. 1994. 163:1309–1313.

13. Evan AP, Willis LR, Connors B, Reed G, McAteer JA, Lingeman JE. Shock wave lithotripsy-induced renal injury. Am J Kidney Dis. 1991. 17:445–450.

14. Evan AP, Willis LR, Lingeman JE, McAteer JA. Renal trauma and the risk of long-term complications in shock wave lithotripsy. Nephron. 1998. 78:1–8.

15. Willis LR, Evan AP, Connors BA, Reed G, Fineberg NS, Lingeman JA. Effects of extracorporeal shock wave lithotripsy to one kidney on bilateral glomerular filtration rate and PAH clearance in minipigs. J Urol. 1996. 156:1502–1506.

16. Newman R, Hackett R, Senior D, Brock K, Feldman J, Sosnowski J, et al. Pathologic effects of ESWL on canine renal tissue. Urology. 1987. 29:194–200.

17. Sheir KZ, Gad HM. Prospective study of the effects of shock wave lithotripsy on renal function: role of post-shock wave lithotripsy obstruction. Urology. 2003. 61:1102–1106.

18. Sheir KZ, Elhalwagy SM, Abo-Elghar ME, Ismail AM, Elsawy E, El-Diasty TA, et al. Evaluation of a synchronous twin-pulse technique for shock wave lithotripsy: a prospective randomized study of effectiveness and safety in comparison to standard single-pulse technique. BJU Int. 2008. 101:1420–1426.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download