Abstract
Purpose
Thoracic dumbbell tumors are relatively rare, usually arising from neurogenic elements. Methods for surgical removal thereof remain controversial. The purpose of this study was to evaluate the surgical results of a single-stage posterior approach with laminectomy and costotransversectomy only for the management of thoracic dumbbell tumors.
Materials and Methods
Eight cases of thoracic large dumbbell tumor were analyzed retrospectively: seven men and one woman (mean age, 49 years). Pathologic findings included schwannoma in five patients, neurofibroma in two patients (Recklinghausen in one patient), and ganglioneuroma in one patient. All patients underwent single-stage removal of dumbbell tumors by a posterior approach followed by laminectomy and costotransversectomy combined with instrumentation. Clinical and radiologic outcomes were reviewed, thereafter.
Results
Operative time ranged from 185 to 420 minutes (mean, 313 minutes), with estimated blood loss ranging from 71 to 1830 mL (mean, 658 mL). Postoperative complications included atelectasis in one case. All patients had tumors successfully removed with no neurological deterioration. Spinal deformities were not observed in any patients at the last follow-up (mean, 52 months), with instrumentation.
Thoracic dumbbell tumors are relatively rare neoplasms that can arise from neurogenic elements within the posterior mediastinum.1,2 They are often very large and involve surrounding structures. Therefore, surgical removal is the treatment of choice and may require an extensive surgical approach. There are various approaches for managing thoracic dumbbell tumors1,3-23 Surgical strategies for managing these tumors depend on the type of tumor according to Eden's classification.10,24 Laminectomy to remove the intraspinal component of a dumbbell tumor should be performed first to prevent spinal cord injury caused by traction and compression when manipulating the tumor.8,9 This is followed by removal of the extraforaminal tumor component.
Although a combined posteroanterior approach has been reported for a large extraforaminal tumor component, this technique necessitates a thoracotomy, two-stages and technical accuracy of the neurosurgeon.10 Furthermore, sternum cleavage may be required for the anterior approach with large, upper thoracic tumors. Hence, it may be more rational to perform a single-stage removal of the thoracic dumbbell tumor without thoracotomy. This report presents eight cases of thoracic dumbbell tumors removal using only a posterior approach and costotransversectomy. We describe the surgical techniques and clinical findings of single-stage removal of these tumors using 3-dimensional computed tomography (3D-CT) to evaluate the surrounding components.
Between 2003 and 2008, 8 patients were admitted for thoracic dumbbell tumor removal at the Department of Orthopedic Surgery in Nagoya University School of Medicine (Table 1). There were seven men and one woman ranging in age from 18 to 73 years (mean age, 49 years). Five patients presented long tract signs with gait disturbance, and 3 patients presented radicular symptoms. The duration of preoperative symptoms ranged from 5 to 72 months (mean duration, 26 months). Preoperative evaluation of each patient included plain radiography of the chest, thoracic-spine and total-spine; magnetic resonance imaging (MRI); and CT including reconstructed 3D, which was able to show involvement with an artery. According to Eden's classification (Table 2),24 five patients were classified as type II, two patients as type III, and one patient as type IV. Five patients underwent CT-guided preoperative biopsy to rule out malignancy, and one patient received preoperative embolization. All patients underwent single-stage removal, which included costotransversectomy and instrumentation. Histopathology revealed schwannoma in five patients, neurofibroma in two patients (Neurofibromatosis type 1 in one patient), and ganglioneuroma in one patient. Extraforaminal tumor extension ranged from 3 to 8.4 cm (mean, 5.6 cm). Involved nerves had to be sacrificed in all patients for complete tumor removal. Follow-up periods after surgery ranged from 24 to 85 months (mean 57 months). We evaluated the severity of a patient's myelopathy before and after surgery according to the Japanese Orthopedic Association (JOA) scoring system. We evaluated postoperative improvement of symptoms using both the recovery ratio of the JOA score and the Hirabayashi method [(postoperative JOA score-preoperative JOA score)/(17-preoperative JOA score)×100%], with a recovery ratio of 100% indicating the best postoperative improvement.25
Patients were placed in the prone position under general anesthesia while somatosensory evoked potentials and motor evoked potentials were monitored. A vertical midline incision was made to expose the laminae bilaterally at designated levels and at the transverse process and rib of the costotransverse joint on the affected side (Fig. 1A). We performed a costotransversectomy on the affected side (Fig. 1B) followed by bilateral laminectomy at the selected level. The dura was opened for intradural tumors (Eden type II) using an operating microscope and extended laterally over the nerve root sleeve. After removing this component of the tumor and sacrificing the entire spinal nerve, we primarily closed the dura in a watertight fashion using fat harvested from subcutaneous tissue with fibrin glue. Then, the extraforaminal tumor with its distal stump and an encapsulated smooth surface tumor at the back side were exposed and carefully enucleated to prevent the need for a thoracotomy (Fig. 1C and D). Finally, we inserted pedicle screws in the first to third thoracic vertebrae, above and below the level of the lesions. We applied contoured rods to prevent deformities due to instability and followed with a bone graft at the decorticated site. Finally, the wound was closed.
Outcomes are documented in Table 1. The mean follow-up period for clinical and radiographic outcome variables was 57 months (range, 24-85 months). Operative time ranged from 185 to 420 minutes (mean, 313 minutes), with estimated blood loss ranging from 71 to 1830 mL (mean, 658 mL). The one case of neurofibromatosis type 1 bled easily. Postoperative complications were pleura injuries during the enucleation of the paravertebral tumor which were able to be repaired. Atelectasis was observed at the opposite side of tumor in one case (No. 3) and was considered to be unrelated with the surgical technique. Tumors were almost totally removed in all cases, as confirmed by MRI. All patients regained the ability to walk 2 or 3 days after surgery. The mean preoperative JOA score was 7.6 (range 4-10). The mean recovery rate was 85.0% (range 66.7-100) at the last follow-up year. Patients experienced no postoperative neurological deterioration, and an MRI at last follow-up revealed no recurrence in any patient. There were no deformities or instability at the fusion areas as determined by plain X-ray and CT.
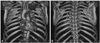
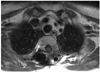
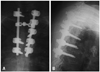
A 55-year-old woman presented to our department with a history of left upper limb and plantar numbness (24 months), which gradually deteriorated. Plain radiography of the chest showed a circular-shaped shadow at the left, upper portion of the lung and a missing left T2 pedicle (Fig. 2). CT and MRI findings were highly suggestive of a dumbbell thoracic cord tumor. An axial view MRI demonstrated intraspinal and extraspinal soft tissue mass at T2-T3 (Fig. 3) with an extraforaminal portion extending 7.4×5×6 cm on the left side. Upon further imaging, 3D-CTs revealed the relationships between the tumor and peritumor structures, i.e., the subclavicular artery, ribs, and vertebrae (Fig. 4).
Thoracic dumbbell tumors are relatively rare.24 Most tumors are benign, neurogenic tumors, with schwannoma, neurofibroma, ganglioneuroma, and neuroblastoma with schwannomas accounting for 90% of all dumbbell tumors.17 Sometimes, the tumors are large by the time they are detected as they grow slowly and are entirely asymptomatic. The extraspinal tumor component is usually larger than the intraspinal component.12
There are various approaches for managing thoracic dumbbell tumors.1,3-23 An important aim of the surgical methods thereof is to remove both the intraspinal and the extraspinal tumor mass. A combined approach and a single posterior approach have been mainly reported. Akwari, et al.12 reported a combination of two approaches. First, a posterior laminectomy was performed by a neurosurgeon, followed by a postero-lateral thoracotomy performed by a thoracic team. This method avoids the risk of bleeding from remnant tumor tissue, compression of the spinal cord, leakage of cerebrospinal fluid and damage to the spinal cord, which can be encountered with two-stage procedures.
Recently, a combined approach involving laminectomy by a neurosurgeon followed by videothoracoscopic removal of the intrathoracic component has been the focus of much interest.4-11 However, it is difficult to precisely use thoracoscopic instruments when there is unexpected bleeding or other emergencies.6 The procedure described in our case report is reasonable for a single surgeon, requiring only a single surgical approach to perform. Postoperative chest tubes have numerous potential complications such as postoperative pain, pulmonary dysfunction and infection.21
For resection of the extraspinal component, a simple enucleation without thoracotomy was performed in the cases reported herein. Moreover, as schwannoma, neurofibroma, and ganglioneuroma tend to encapsulate well,26,27 enucleation, i.e., intracapsular manipulation, from the posterior approach is not difficult for a surgeon to perform, and there is less of a chance of injury to peritumoral structures such as an artery. The most serious complication that can develop during this surgery is spinal cord ischemia due to injury to the Adamkiewicz artery. However, we do not touch the aorta or segmental artery during surgery. Although the extraspinal component, including the aorta, cannot be fully seen, enucleation is a safer method since ablation with aorta is unnecessary. The goal of surgery is to remove the tumor entirely, ameliorating symptoms and eliminating recurrence in surrounding structures. Malignant alteration is rarely described in the literature. Although very rarely, there have been reports of malignant transformation.28,29 Even though we detected no recurrences in our patients, we need to follow by radiographic evaluation after the surgery for a long time.
Advocates of a combined approach claim that the posterior approach is restricted to a vertical midline incision centered over the tumor and a transthoracic transpleural approach requires removal of fewer facet joints, transverse processes, and ribs.6,8 For these reasons, several authors hypothesized that segmental stability may be less compromised with a combined approach than a purely posterior approach with costotransversectomy.6,8,18
Vecil, et al.22 reported multi-level rib resections and laminectomy necessitated posterior spinal stabilization. Agrawal, et al.30 reported on single stage excision with the posterior approach without instrumentation. We believe, however, that for safer surgery, intra- and large extraspinal lesions connected through the foramen should not be resected without facetectomy and costotransversectomy, considering partial facetectomy with scalloping lesions has the risk for postoperative instability. Therefore, spinal instrumentation with pedicle screws and bone grafting was additionally performed in these reported cases to prevent deformity. There were no complications such as instrumentation failures or adjacent segmental disorders at last follow-up.
Takamura, et al.18 reported that it is essential to individualize preoperative surgical strategies to each patient. In all cases, surgical strategies were devised preoperatively supported by 3D-CT images revealing the relationships between tumors and peritumoral structures, such as arteries, ribs, and vertebrae.
In this study, a large tumor size of 8.4 cm was able to be removed. However, it is not considered appropriate to remove tumors with the posterior approach if malignancy is suspected.
In conclusion, single-stage surgery with laminectomy and costotransversectomy may be a useful method for removing thoracic dumbbell tumors.
Figures and Tables
Fig. 1
(A) A vertical midline incision was made to expose the laminae bilaterally at designated levels and at the transverse process and rib of the costotransverse joint on the affected side. (B) Costotransversectomy at the affected side was resected. (C) An encapsulated smooth surface tumor at the back side was exposed and enucleated to prevent the need for thoracotomy. (D) An enucleated tumor.
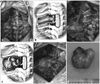
Fig. 2
Plain radiography of the chest revealed a circular-shaped shadow of the left, upper portion of the lung and left T2 pedicle sign.
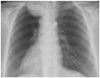
Fig. 3
MRI showing coronal and axial views of the intraspinal and extraspinal soft tissue mass at T2-T3.
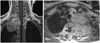
Fig. 4
3D-CT showing the relationships between the tumor and peritumoral structures, that is, the aorta, ribs, and vertebrae. (A) From anterior side. (B) From posterior side. 3D-CT, 3-dimensional computed tomography.
References
1. Ishida T, Maruyama R, Saitoh G, Mitsudomi T, Sugimachi K. Thoracoscopy in the management of intrathoracic neurogenic tumors. Int Surg. 1996. 81:347–349.
2. Liu HP, Yim AP, Wan J, Chen H, Wu YC, Liu YH, et al. Thoracoscopic removal of intrathoracic neurogenic tumors: a combined Chinese experience. Ann Surg. 2000. 232:187–190.

3. Grillo HC, Ojemann RG, Scannell JG, Zervas NT. Combined approach to "dumbbell" intrathoracic and intraspinal neurogenic tumors. Ann Thorac Surg. 1983. 36:402–407.

4. Shadmehr MB, Gaissert HA, Wain JC, Moncure AC, Grillo HC, Borges LF, et al. The surgical approach to "dumbbell tumors" of the mediastinum. Ann Thorac Surg. 2003. 76:1650–1654.

5. Heltzer JM, Krasna MJ, Aldrich F, McLaughlin JS. Thoracoscopic excision of a posterior mediastinal "dumbbell" tumor using a combined approach. Ann Thorac Surg. 1995. 60:431–433.

6. Vallières E, Findlay JM, Fraser RE. Combined microneurosurgical and thoracoscopic removal of neurogenic dumbbell tumors. Ann Thorac Surg. 1995. 59:469–472.

7. Fiumara E, D'Angelo V, Florio FP, Nardella M, Bisceglia M. Preoperative embolization in surgical treatment of spinal thoracic dumbbell schwannoma. A case report. J Neurosurg Sci. 1996. 40:153–156.
8. Citow JS, Macdonald RL, Ferguson MK. Combined laminectomy and thoracoscopic resection of a dumbbell neurofibroma: technical case report. Neurosurgery. 1999. 45:1263–1265.

9. Dickman CA. Comments in combined laminectomy and thoracoscopic resection of a dumbbell neurofibroma: technical case report. Neurosurgery. 1999. 45:1266.
10. Konno S, Yabuki S, Kinoshita T, Kikuchi S. Combined laminectomy and thoracoscopic resection of dumbbell-type thoracic cord tumor. Spine (Phila Pa 1976). 2001. 26:E130–E134.

11. Jules JA, Guarnieri JM, Alkofer B, Le Rochais JP, Icard P. Posterior intrathoracic neurinoma cure: a transforaminal resection after a thoracotomy. Ann Thorac Surg. 2005. 79:1411–1412.

12. Akwari OE, Payne WS, Onofrio BM, Dines DE, Muhm JR. Dumbbell neurogenic tumors of the mediastinum. Diagnosis and management. Mayo Clin Proc. 1978. 53:353–358.
13. Joseph SG, Tellis CJ. Posterior mediastinal mass with intraspinous extension. Chest. 1988. 93:1101–1103.

14. Shamji FM, Todd TR, Vallières E, Sachs HJ, Benoit BG. Central neurogenic tumours of the thoracic region. Can J Surg. 1992. 35:497–501.
15. Lucas S, Cendan E, Auque J, Civit T, Caremelle S, Braun D. [Asymptomatic giant thoracic dumbbell neurinoma. Apropos of a case]. J Chir (Paris). 1992. 129:81–87.
16. Yüksel M, Pamir N, Ozer F, Batirel HF, Ercan S. The principles of surgical management in dumbbell tumors. Eur J Cardiothorac Surg. 1996. 10:569–573.

17. McCormick PC. Surgical management of dumbbell and paraspinal tumors of the thoracic and lumbar spine. Neurosurgery. 1996. 38:67–74.

18. Takamura Y, Uede T, Igarashi K, Tatewaki K, Morimoto S. Thoracic dumbbell-shaped neurinoma treated by unilateral hemilaminectomy with partial costotransversectomy--case report. Neurol Med Chir (Tokyo). 1997. 37:354–357.

19. Onesti ST, Ashkenazi E, Michelsen WJ. Transparaspinal exposure of dumbbell tumors of the spine. Report of two cases. J Neurosurg. 1998. 88:106–110.
20. Miura J, Doita M, Miyata K, Yoshiya S, Kurosaka M, Yamamoto H. Horner's syndrome caused by a thoracic dumbbell-shaped schwannoma: sympathetic chain reconstruction after a one-stage removal of the tumor. Spine (Phila Pa 1976). 2003. 28:E33–E36.

21. Payer M, Radovanovic I, Jost G. Resection of thoracic dumbbell neurinomas: single postero-lateral approach or combined posterior and transthoracic approach? J Clin Neurosci. 2006. 13:690–693.

22. Vecil GG, McCutcheon IE, Mendel E. Extended lateral parascapular approach for resection of a giant multi-compartment thoracic schwannoma. Acta Neurochir (Wien). 2008. 150:1295–1300.

23. Murovic JA, Charles Cho S, Park J. Surgical strategies for managing foraminal nerve sheath tumors: the emerging role of CyberKnife ablation. Eur Spine J. 2010. 19:242–256.

25. Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976). 1981. 6:354–364.

26. Radulovi DV, Branislav D, Skender-Gazibara MK, Igor NM. Cervical dumbbell ganglioneuroma producing spinal cord compression. Neurol India. 2005. 53:370–371.

27. Sasaki K, Kohno T, Mun M, Yoshiya T. Thoracoscopic removal of middle mediastinal schwannoma originating from recurrent nerve. Thorac Cardiovasc Surg. 2008. 56:375–377.

28. Martins MD, Anunciato de Jesus L, Fernandes KP, Bussadori SK, Taghloubi SA, Martins MA. Intra-oral schwannoma: case report and literature review. Indian J Dent Res. 2009. 20:121–125.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download