Abstract
Materials and Methods
We used generic health status and general psychological health questionnaires to determine the range of issues that needed to be considered in examining the burden of common variable immunodeficiency (CVID).
Results
The health status of patients with CVID was lower than that observed in normal subjects. Overall, Role-Physical and General Health scales correlated with a poorer clinical status. Surprisingly, the duration of disease did not influence health status. Being female, older, General Health Questionnaire-positive and alexithymic proved to be major risk factors associated with a poor health status. Patients with chronic lung disease and chronic diarrhea had the lowest values on the Medical Outcome Study, Short Form SF-36 (SF-36) scales. Disease severity perception was associated with the General Health Questionnaire and alexithymia status. Limitations in daily activities as a result of lower physical health were the major problems facing common variable immunodeficiency patients.
Common variable immunodeficiency (CVID) is the most frequent symptomatic antibody deficiency, characterized by low levels of serum immunoglobulins and impaired antibody response.1-3 CVID may occur at any age, demonstrating predominance in the adult age group. The clinical spectrum includes respiratory infections, gastrointestinal diseases, autoimmune diseases, granulomatous manifestations, and cancers.4,5 Long-term administration of immunoglobulins reduces the incidence of infections. However, despite IgG replacement, CVID patients may still suffer from respiratory infections, caused by encapsulated bacteria, leading to permanent lung damage.6,7 Quantifying the health related quality of life (HRQoL) in primary immunodeficiency conditions has relatively recently began as an effort to document the outcomes of therapeutic intervention, and in order to do so, investigators have begun to use generic measures such as the Medical Outcome Study, Short Form SF-368/SF-129 or Life Quality Index.10 The aims of our study were to determine the range of issues that need to be considered in examining the burden of CVID and to evaluate HRQoL and psychological status of patients attending our day-hospital Unit, using a generic health status questionnaire and general psychological health questionnaires. Moreover, we intended to compare the evaluations of disease severity from both the patient and the physician perspectives. The underlying hypothesis was that CVID patients have a low HRQoL and, according to the global severity assessment of his/her own disease, mental and psychological aspects, which contribute to the burden of the disease, should be considered. Moreover, we investigated if risks of depression/anxiety and alexithymia could be associated with different perceptions of disease severity and health status.
For this observational, short-term longitudinal cohort study, performed in a day hospital setting, patients' participation was obtained via signed informed consent. The project was approved by our Institute Ethical Committee. The study was conducted during the period of January-October 2010. HRQoL was tested twice in a six month period. The entry criteria for this study were: 1) a diagnosis of CVID and 2) a clinical follow-up of at least five years at our center for primary immunodeficiencies. CVID diagnosis was made in patients over two years of age by approved criteria;2 including low levels of serum IgG, IgA, and/or IgM; antibody deficiency with impaired response to tetanus and pneumococcal antigen immunization; peripheral B cell numbers of less than 2%; and exclusion of hypogammaglobulinemia due to other primary or secondary immunodeficiencies. Detailed information on personal data, date of diagnosis, immunological data and clinical manifestations, as well as route and dosage of immunoglobulin replacement were collected on a yearly basis from the initial diagnosis via a questionnaire filled in by a physician of our center. For each patient, complete blood counts, lymphocyte subsets, chemistries, serum immunoglobulin levels, and culture tests were performed four times per year. Chest and sinus computerized tomography (CT) scans were performed every four years, and gastrointestinal endoscopy with biopsy was performed every two years. All data were processed in a database and sent to the Interuniversity Computing Centre responsible for processing and analyzing the data. All 112 adult CVID patients referred to our center were recruited, and 16 refused to participate. Data were analyzed on the same day of their periodical clinical assessment. The ninety-six patients (50 males and 46 females) who agreed to participate to our study had a mean age of 48.2±17 years. Fifty-two patients were less than 50 years of age (group 1) and 44 were older than 50 years (group 2). The mean age at diagnosis was 36±17.5 years. The mean duration of disease since diagnosis was 10.7 years (range 5-36). The majority of patients (87.5%) were receiving intravenous gammaglobulin every 2 or 3 weeks in a day hospital setting. Twelve patients self-infused subcutaneous immunoglobulins at home with a monthly clinical follow-up at our out-patient service. Before clinical examination and immunoglobulin therapy, patients were asked to fill in the four questionnaires concerning their HRQoL, general health status, and the possible presence of depression/anxiety and alexithymia.
Although designed as a generic health status indicator for use in population surveys and health policy evaluation studies, the SF-36 (8) can also be used as an outcome measure. The SF-36 includes 36 items in a Likert-type or forced-choice format, intended to measure the following eight dimensions: physical functioning (PF, limitations in performing physical activities such as bathing or dressing), role-physical (RP, limitations in work and other daily activities as a result of physical health), bodily pain (BP, how severe and limiting is pain), general health (GH, how general personal health is perceived by the patient), vitality (VT, feeling tired and worn out vs. feeling energetic), social functioning (SF, interference with normal social activities due to physical or emotional problems), role-emotional (RE, limitations in work and other daily activities as a result of emotional problems), and mental health (MH, feeling nervous and depressed vs. peaceful, happy and calm). Scores for each domain range from 0 to 100, with higher scores indicating better health status. Two additional summary measures, the physical (PCS) and mental component scores (MCS), cross-culturally validated in the framework of the International Quality of Life Assessment project for the Italian version of the SF-36,11 were also obtained. As controls for general health status, we used the Italian Normative Values.11
The General Health Questionnaire-1212 is a self-administered 12-item questionnaire designed to measure psychological distress and to detect current non-psychotic psychiatric disorders, such as depression and anxiety. The reliability and validity of the Italian version have been tested in several diseases, including dermatological conditions.13 Answers are given on a four-point scale; for instance, the item "in the last weeks, did you feel under strain?" invites the following answers: "no", "not more than usual", "more than usual", and "much more than usual". When scored with the binary method (0-0-1-1), the GHQ-12 can be used as a screening tool to detect minor non-psychotic psychiatric disorders, yielding final scores that range from 0 to 12. Operationally, patients scoring 4 or more were considered as "GHQ-positive" (GHQ+): at risk of anxiety/depression.
The 20-item Toronto Alexithymia Scale (TAS-20) questionnaire14,15 was used to evaluate alexithymia, i.e., the difficulty in identifying and describing feelings. It gives three subscale scores, measuring, respectively, the difficulty in identifying feelings, the difficulty in describing and communicating feelings, and the tendency to focus on the concrete details of external events rather than on feelings, fantasies, and other aspects of one's own inner experience ("externally oriented thinking"). There is evidence of internal consistency, test-retest reliability and factorial validity for the Italian version of the TAS-20.16 The item ratings under each TAS-20 scale were added together to give subscale scores. Then, these scores were summed to give the total score. The classical cut-offs are: below 52, people are classified as non-alexithymic; between 52 and 60, borderline alexithymic; and more than 60, alexithymic.
For each patient, an overall clinical severity evaluation of the disease was given by the physician and by the patient him/herself. The Physician Global Assessment (PhGA) and the Patient Global Assessment (PtGA) comprised the questions, "In your opinion, compared to other patients with the same condition, how severe is the disease of patient X?" and "In your experience, how severe is your disease?", respectively. Answers were given on a 5-point scale: "very mild", "mild", "moderate", "severe", and "very severe". The physician recorded the evaluation at the end of the visit, and the patients recorded their evaluations after completing the other questionnaires.
In order to compare PtGA and PhGA, we created three categories: "Agreement", PtGA was equal to PhGA; "Patient underestimates", PtGA was lower than PhGA; "Patient overestimates", PtGA was higher than PhGA. Cohen's kappa was calculated to measure the agreement between the evaluations of severity by both patients and physicians. A value of 1 indicated perfect agreement, while a value of 0 indicated that agreement was no better than chance. After univariate analyses, two logistic regression models were tested in order to investigate the possible determinants of underestimation or overestimation of severity by the patient. The dependent variables were, respectively, "patient underestimates" and "patient overestimates" vs. others. Independent variables were: gender, age (dichotomised cut-off of 50 years), GHQ-12 (case/non-case), TAS-20 (alexithymia/no-alexithymia). For all subjects, sex, age, duration of disease, presence of chronic lung disease, chronic sinusitis and chronic diarrhea, were compared using Wilcoxon-Mann-Whitney tests. Quality of life scores eight areas, and the overall physical and mental scores were calculated based on the SF-36 manual. Then, one-sample z-test was used to compare our study group with the general Italian population. To internally compare the overall physical and mental quality of life scores in various subgroups in our CVID study group, the Wilcoxon-Mann-Whitney test was used. Statistical analyses included the t-test for independent samples and the chi-square test as well as a logistic regression analysis to assess the independent role of factors of interest in determining poor HRQoL. All analyses were performed using the Stata version 11 software.
Forty-two percent of patients were affected by chronic sinusitis at the time of the study. A high prevalence (65.6%) of patients with chronic lung disease (CLD) and bronchiectasis, confirmed by chest CT scanning, was present at the time of the survey. The prevalence was higher than 50% for both age groups. The 63 individuals with CLD were slightly older (mean age 49.8±17) than those without CLD (mean age 43.8±15.6); 76% of the subjects with CLD had one or more episodes of acute bronchitis or acute sinusitis during the study period. One of the subjects with CLD was using oxygen. Two patients died during the study period, one for non-Hodgkin B cell lymphoma and one for post-splenectomy sepsis. Twenty-two percent of the patients with chronic lung disease were taking daily prophylactic antibiotics (20). Chronic diarrhea was observed in 22.4% of patients. The percentage of affected patients was higher in the older age group (>50 years of age). Twenty-one patients (22.6%) had CLD, chronic diarrhea and sinusitis at the same time. We found no difference in the prevalence of chronic diseases between males and females. Other medical problems commonly seen in adults including hypertension and type II diabetes were observed in 18% and 9% of patients, respectively.
The SF-36 mean values of all CVID patients and of different age groups (25-34 years; 35-44 years; 45-54 years; 55-64 years) were compared to the normative data of the Italian population (Fig. 1). Health status in CVID patients was statistically significant lower than that observed in normal subjects. The mental domain scales (Vitality, Social Functioning, Role-Emotional, Mental Health) had values close to those reported in normal subjects in all age groups, whereas the physical mean value scales (Physical Functioning, Role-Physical, Bodily Pain, General Health) were lower than the normative data (p<0.05). Role-Physical and General Health scales showed the lowest values among CVID patients, overall. Patients with CLD (p<0.05) and chronic diarrhea (p<0.05) had lower values on SF-36 scales with respect to patients with no chronic diseases, while chronic sinusitis had no effect on HRQoL (Table 1). No correlations were found for IgG serum levels and health status assessed by PCS and MCS or by GHQ-12 (computed using the classical Likert score). We found no differences in HRQoL between patients on intravenous immunoglobulin therapy (IVIG) and patients who self-administered subcutaneous immunoglobulins (SCIG) as an in-home therapy (data not shown).
In our study, trough IgG levels did not correlate with frequency of respiratory infections, which agrees with the data of a recently published multicenter prospective study.17 For the multivariate analysis performed in order to determine independent predictors of CVID-associated clinical conditions, we did not find an increased risk of pneumonia, bronchiectasis, or acute and chronic sinusitis in patients who had different IgG trough levels, except for those who had IgG trough levels below 400 mg/dL.17
Female, older, GHQ-positive and alexithymic individuals had statistically significant lower mean SF-36 scale values (p<0.05) compared to male, younger, GHQ-negative, non-alexithymic individuals (Table 1). GHQ-positive patients (at risk of minor psychiatric non psychotic diseases such as depression/anxiety) or alexithymic individuals (who have difficulty in identifying and describing feelings) showed the worst values for evaluating QoL. SF-36 summary measures for physical and mental scales (PCS-MCS) demonstrated that GHQ-positive patients had lower mean values for MCS (34±13) in comparison with GHQ-negative patients (47±10) (p<0.05), while alexithymic individuals had lower values for PCS (33±7) in comparison to non-alexithymic individuals (42±13) (p<0.05) and lower values for MCS (36±12) in comparison to non-alexithymic individuals (45±12) (p<0.05).
Cohen's kappa was calculated to measure the agreement between severity evaluations by the patients and by physicians (0.16). PhGA was greater in alexithymic CVID patients and GHQ-positive CVID patients (57% and 48%, respectively) (Table 1). Only 15% of alexithymic individuals and only 25% of GHQ-positive patients considered their disease severity as "high". With a logistic regression analysis, considering "underestimation" of the disease severity by the patient as the dependent variable and gender, age, GHQ-positive status, and presence of alexithymia as independent variables, a statistically significant result was only observed for those less than 50 years of age (p=0.045). For "overestimation", no variables were statistically significant; however, GHQ-positive patients had an OR of 2 (95% CI 0.6-6.9).
A second patient assessment was performed over a six-month period using the same questionnaires in order to confirm the results obtained at the first assessment. The results observed in the first and second assessment of the mean values of SF-36 summary measures allowed for identification of patients who reported a change in their GHQ status. The percentage of GHQ-positive patients was 27% at the first observation and 29% at the second observation. As expected, in subjects who had an increase in GHQ score, we observed a reduction in MCS values as a result of worsening mental health.
HRQoL is a subjective perception of health status (including disease and treatment) concerning physical, psychological, and social functioning and wellbeing. When considering the burden of a disease, the health domain is the most pertinent aspect of quality of life and on which this study focused. Self-completed measures, acceptable to patients, are available for use in clinical settings with adequate sensitivity and specificity in their ability to identify problems and changes. Moreover, patient-reported outcome measures in clinical practice have been proposed as a means of facilitating doctor-patient communication, uncovering patients' problems, monitoring disease or treatment, and screening for functional problems.18-21 However, while HRQoL measures are now quite commonly included in the protocols of randomized controlled clinical trials and other clinical studies, their use in routine clinical practice is still quite limited. Only a few studies have analyzed the HRQoL of life in patients with primary immunodeficiencies.22-25 A detailed analysis of the burden of CVID, besides the problems of immunoglobulin treatment, is lacking. We examined the possibility that the quality of life may be influenced by the ages of the patients; the length of CVID disease; the presence or absence of chronic sinusitis, chronic lung disease, or chronic diarrhea; and whether the subject received gammaglobulin therapy at home or in a hospital setting. The duration of disease did not influence health status, while the most seriously affected HRQoL measures were due to the presence of chronic clinical conditions. Respiratory tract infections were the most prominent clinical problem observed in our patients, in agreement with all published data on CVID patients (reviewed in 3). The strong impact of CLD on HRQoL was in line with what has been reported in patients with chronic obstructive pulmonary disease26-28 and in patients with inflammatory bowel diseases29 where a relationship between the number of symptoms and impaired levels on both physical and mental scales has been observed. Due to their chronic clinical condition, CVID patients are faced with the prospect of lifelong IgG replacement treatment administered intravenously or subcutaneously at the hospital or at home. We found no differences in HRQoL between patients on IVIG and patients who self-administered SCIG at home. This was in contrast with the observation that adults on IVIG therapy showed improvements in HRQoL (Vitality, Mental Health, and Social Functioning) after switching to SCIG home therapy.24 This discrepancy might be explained by the low number of patients who decided to shift from IVIG to SCIG and to the different measures used in our study to investigate the burden of CVID. In fact, according to our observation, other important aspects, besides those measured by SF-36, might have influenced the comprehensive evaluation of patients' status, such as their psychological condition. Disorders such as anxiety and depression are particularly prevalent in hospital settings and still often go unrecognized.30,31 Moreover, patients with a more severe perception of the disease (PtGA) and patients who were judged by the physician (PhGA) as seriously affected reported a lower health status than the others.
Our data underlined the importance of conducting a periodical Health related quality of life assessment on patients with primary antibody deficiencies and other chronic illnesses. In fact, as expected, patients with chronic lung disease and chronic diarrhea had the lowest health related quality of life values; however, being female, older, General Health Questionnaire-positive and alexithymic also proved to be major risk factors associated with a poor health status. Finally, we suggest that in a comprehensive evaluation of HRQoL assessment should be performed in each patient in order to document the outcomes of clinical management and therapeutic intervention. Moreover, we confirmed that it is necessary to care for at risk patients with psychological support throughout their lifetime.
ACKNOWLEDGEMENTS
This study has been funded by the EU Commission, EURO-PADnet Health-F2-2008-201549 and supported by Jeffrey Modell Fondation and Fondazione Eleonora Lorillard Spencer Cenci. We would like to thank our patients, nurses (Anna Rita Ligia, Anna Petroni, Francesca Iasi, Michele Tucci, Assunta Iannucci) for their continuous and precious collaboration. We would also like to thank the Italian patient's association AIP (Associazione Immunodeficienze Primitive).
References
1. International Union of Immunological Societies. Primary immunodeficiency diseases. Report of an IUIS Scientific Committee. Clin Exp Immunol. 1999; 118(Suppl 1):1–28.
2. Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999; 93:190–197. PMID: 10600329.
3. Park MA, Li JT, Hagan JB, Maddox DE, Abraham RS. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008; 372:489–502. PMID: 18692715.

4. Conley ME, Park CL, Douglas SD. Childhood common variable immunodeficiency with autoimmune disease. J Pediatr. 1986; 108:915–922. PMID: 2423668.

5. Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999; 92:34–48. PMID: 10413651.

6. Kainulainen L, Varpula M, Liippo K, Svedström E, Nikoskelainen J, Ruuskanen O. Pulmonary abnormalities in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol. 1999; 104:1031–1036. PMID: 10550749.

7. Quinti I, Soresina A, Spadaro G, Martino S, Donnanno S, Agostini C, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol. 2007; 27:308–316. PMID: 17510807.

8. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992; 30:473–483. PMID: 1593914.
9. Howard V, Greene JM, Pahwa S, Winkelstein JA, Boyle JM, Kocak M, et al. The health status and quality of life of adults with X-linked agammaglobulinemia. Clin Immunol. 2006; 118:201–208. PMID: 16377251.

10. Daly PB, Evans JH, Kobayashi RH, Kobayashi AL, Ochs HD, Fischer SH, et al. Home-based immunoglobulin infusion therapy: quality of life and patient health perceptions. Ann Allergy. 1991; 67:504–510. PMID: 1958004.
11. Apolone G, Mosconi P. The Italian SF-36 Health Survey: translation, validation and norming. J Clin Epidemiol. 1998; 51:1025–1036. PMID: 9817120.
12. Goldberg D. The detection of psychiatric illness by questionnaire. 1972. London: Oxford University Press.
13. Picardi A, Abeni D, Pasquini P. Assessing psychological distress in patients with skin diseases: reliability, validity and factor structure of the GHQ-12. J Eur Acad Dermatol Venereol. 2001; 15:410–417. PMID: 11763380.

14. Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. J Psychosom Res. 1994; 38:23–32. PMID: 8126686.

15. Bagby RM, Taylor GJ, Parker JD. The Twenty-item Toronto Alexithymia Scale--II. Convergent, discriminant, and concurrent validity. J Psychosom Res. 1994; 38:33–40. PMID: 8126688.

16. Bressi C, Taylor G, Parker J, Bressi S, Brambilla V, Aguglia E, et al. Cross validation of the factor structure of the 20-item Toronto Alexithymia Scale: an Italian multicenter study. J Psychosom Res. 1996; 41:551–559. PMID: 9032718.

17. Quinti I, Soresina A, Guerra A, Rondelli R, Spadaro G, Agostini C, et al. Effectiveness of immunoglobulin replacement therapy on clinical outcome in patients with primary antibody deficiencies: results from a multicenter prospective cohort study. J Clin Immunol. 2011; 31:315–322. PMID: 21365217.

18. Higginson IJ, Carr AJ. Measuring quality of life: using quality of life measures in the clinical setting. BMJ. 2001; 322:1297–1300. PMID: 11375237.

19. Lohr KN. Advances in health status assessment. Overview of the conference. Med Care. 1989; 27(3 Suppl):S1–S11. PMID: 2921881.
20. Lohr KN. Applications of health status assessment measures in clinical practice. Overview of the third conference on advances in health status assessment. Med Care. 1992; 30(5 Suppl):MS1–MS14. PMID: 1583924.
21. Sweinberg SK, Wodell RA, Grodofsky MP, Greene JM, Conley ME. Retrospective analysis of the incidence of pulmonary disease in hypogammaglobulinemia. J Allergy Clin Immunol. 1991; 88:96–104. PMID: 2071789.

22. Nicolay U, Kiessling P, Berger M, Gupta S, Yel L, Roifman CM, et al. Health-related quality of life and treatment satisfaction in North American patients with primary immunedeficiency diseases receiving subcutaneous IgG self-infusions at home. J Clin Immunol. 2006; 26:65–72. PMID: 16418804.

23. Tcheurekdjian H, Palermo T, Hostoffer R. Quality of life in common variable immunodeficiency requiring intravenous immunoglobulin therapy. Ann Allergy Asthma Immunol. 2004; 93:160–165. PMID: 15328676.

24. Gardulf A, Borte M, Ochs HD, Nicolay U. Vivaglobin Clinical Study Group. Prognostic factors for health-related quality of life in adults and children with primary antibody deficiencies receiving SCIG home therapy. Clin Immunol. 2008; 126:81–88. PMID: 17964220.

25. Winkelstein JA, Conley ME, James C, Howard V, Boyle J. Adults with X-linked agammaglobulinemia: impact of disease on daily lives, quality of life, educational and socioeconomic status, knowledge of inheritance, and reproductive attitudes. Medicine (Baltimore). 2008; 87:253–258. PMID: 18794707.
26. Carrasco Garrido P, de Miguel Díez J, Rejas Gutiérrez J, Centeno AM, Gobartt Vázquez E, Gil de Miguel A, et al. Negative impact of chronic obstructive pulmonary disease on the health-related quality of life of patients. Results of the EPIDEPOC study. Health Qual Life Outcomes. 2006; 4:31. PMID: 16719899.

27. Bridevaux PO, Gerbase MW, Probst-Hensch NM, Schindler C, Gaspoz JM, Rochat T. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax. 2008; 63:768–774. PMID: 18505800.

28. Voll-Aanerud M, Eagan TM, Wentzel-Larsen T, Gulsvik A, Bakke PS. Respiratory symptoms, COPD severity, and health related quality of life in a general population sample. Respir Med. 2008; 102:399–406. PMID: 18061422.

29. Reilly MC, Gerlier L, Brabant Y, Brown M. Validity, reliability, and responsiveness of the work productivity and activity impairment questionnaire in Crohn's disease. Clin Ther. 2008; 30:393–404. PMID: 18343277.

30. Gilbody SM, House AO, Sheldon TA. Routinely administered questionnaires for depression and anxiety: systematic review. BMJ. 2001; 322:406–409. PMID: 11179161.

31. van Hemert AM, Hengeveld MW, Bolk JH, Rooijmans HG, Vandenbroucke JP. Psychiatric disorders in relation to medical illness among patients of a general medical out-patient clinic. Psychol Med. 1993; 23:167–173. PMID: 8475204.

Fig. 1
SF-36 scales, mean values in (A) CVID patients vs. normal subjects - Italian normative. (B) CVID patients vs. normal subjects (25-34 years). (C) CVID patients vs. normal subjects (35-44 years). (D) CVID patients vs. normal subjects (45-54 years). (E) CVID patients vs. normal subjects (55-64 years). Higher scores denote better health SF-36 scales. PF, physical functioning (limitations in performing physical activities such as bathing or dressing); RP, role-physical (limitations with work and other daily activities as a result of physical health); BP, bodily pain (how severe and limiting is pain); GH, general health (how general personal health is evaluated by the patient); VT, vitality (feeling tired and worn out vs. feeling full of energy); SF, social functioning (interference with normal social activities due to physical or emotional problems); RE, role-emotional (limitations with work and other daily activities as a result of emotional problems); MH, mental health (feeling nervous and depressed vs. peaceful, happy and calm). CVID, common variable immunodeficiency; SF-36, Shot From-36. Normal subjects, Italian Normative11). *p<0.05 t-test.
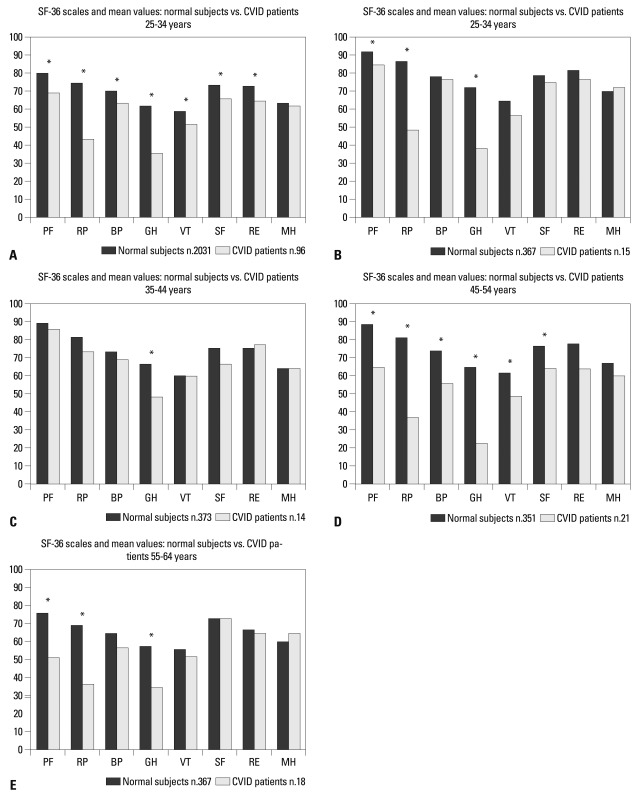
Table 1
SF-36 and Alexithymia Mean Values and Standard Deviation for Clinical Characteristics of CVID Patients*
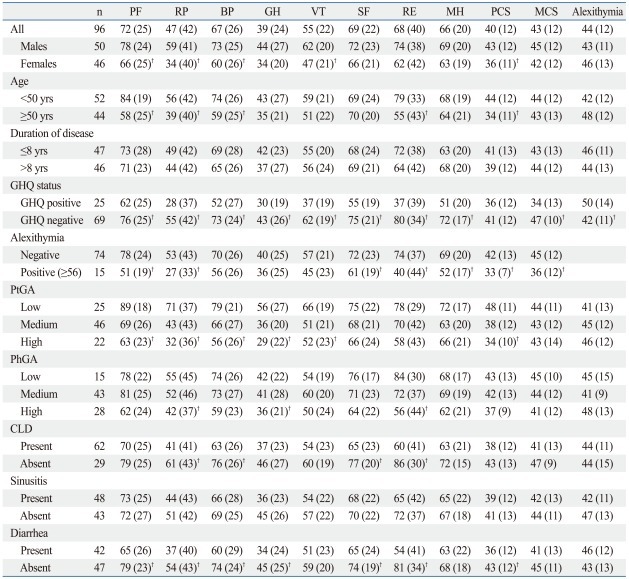
SF-36: PF, physical functioning; RP, role-physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role-emotional; MH, mental health; PCS, physical component summary; MCS, mental component summary; CLD, chronic lung disease; PhGA, physician global assessment; PtGA, patient global assessment; CVID, common variable immunodeficiency; GHQ, General Health Questionnaire.
*p<0.05.
†Totals may vary because of missing values.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download