Abstract
Purpose
Tubulointerstitial hypoxia in the kidney is considered a hallmark of injury and a mediator of the progression of tubulointerstitial fibrosis. Hypoxia-inducible factor-1alpha (HIF-1alpha), a master transcription factor in cellular adaptation to hypoxia, regulates a wide variety of genes, some of which are closely associated with tissue fibrosis. The present study set out to characterize urinary HIF-1alpha expressions in patients with lupus nephritis (LN) and to explore whether urinary HIF-1alpha expressions are associated with histologic chronicity changes and renal function.
Materials and Methods
Urinary HIF-1alpha levels were measured by enzyme-linked immunosorbent assays in 42 patients with LN and in 30 healthy controls. Activity and chronicity indexes as well as tubular HIF-1alpha expressions were analyzed for each specimen.
Results
Urinary HIF-1alpha levels were higher in LN patients than in healthy controls (3.977±1.696 vs. 2.153±0.554 ng/mL, p<0.001) and were associated with histologic chronicity indexes (r=0.463, p<0.01) and eGFR (r=-0.324, p<0.05). However, urinary HIF-1alpha levels showed no correlation with histologic activity indexes, anti-dsDNA, ANA, complement 3 and 4 levels, proteinuria, systemic lupus erythematosis disease activity index, and WHO pathological classification (p>0.05).
Lupus nephritis (LN) is one of the main causes of morbidity and mortality in systemic lupus erythematosus (SLE).1 Although the glomerular abnormalities of LN have attracted much attention, the mechanisms that lead to tubulointerstitial fibrosis and damage in LN remain obscure. Tubulointerstitial injury is characterized by tubular atrophy and interstitial fibrosis.2 Pathological analysis shows that the extent of tubulointerstitial damage is better correlated with impaired renal function than the degree of glomerular injury in patients with chronic kidney disease (CKD) and it may be less amenable to treatment compared with glomerular lesions.3
Accumulating evidence emphasizes chronic hypoxia in the tubulointerstitium as a final common pathway to end-stage renal disease (ESRD), and the final common pathway of chronic hypoxia operates principally in the tubulointerstitium.4,5 Renal hypoxia in early and late stage of CKD is secondary to imbalances in vasoactive factors, obliteration and loss of the interstitial capillary, reduced efficiency of oxygen diffusion, increased oxygen demand from hyperfiltration and tubular hypertrophy.5-9 Recent evidence shows that hypoxia may accelerate CKD progression by affecting renal cell survival, inflammatory cell infiltration, ECM deposition, and epithelial to mesenchymal transition (EMT).6,7,10,11
The family of hypoxia-inducible factors (HIFs) are central regulators of hypoxic responses.12 HIFs regulate several target genes that have important functions in renal physiologic and pathophysiologic processes, including energy metabolism, vasomotor regulation, angiogenic growth, matrix metabolism, and apoptosis/cell survival.13-17 HIF-1 is a heterodimer composed of a constitutively expressed HIF-1β subunit and an O2-regulated HIF-1alpha subunit. HIF-1alpha plays a central role in the hypoxic response of tubular epithelial cells.10 Indeed, hypoxia-inducible gene expression in primary renal proximal tubular epithelial cells is almost completely blocked by inactivation of HIF-1alpha, suggesting that their response to hypoxia is largely dependent on HIF-1alpha.18 In addition, genetic ablation of HIF-1alpha inhibited the development of tubulointerstitial fibrosis in vivo.19 In summary, HIF-1alpha appears to be a critical contributor to the progression of tubulointerstitial fibrosis due to hypoxia.
In this study, we investigated urinary HIF-1alpha levels in patients with LN and analyzed the associations between urinary HIF-1alpha levels and the clinical features in LN patients.
Forty-two patients with LN who underwent renal biopsy at Hainan Provincial People's Hospital from 2003 January to 2010 December were identified via an electronic medical record system. All 42 patients fulfilled at least four of the 1997 American College of Rheumatology revised criteria for SLE and had persistent proteinuria greater than 0.5 g/day or cellular casts, as described in the criteria for renal involvement.20 This study was approved by the Institutional Review Board of Hainan Provincial People's Hospital and informed consent from all patients was obtained.
Kidney specimens from 42 patients with LN were stained with hematoxylin-eosin and then classified according to the International Society of Nephrology/Renal Pathology Society 2003 classification.21 Activity indices (AI) and chronicity indices (CI) were calculated in proliferative forms.22 Quantitation of interstitial inflammation and chronic tubulo-interstitial damage (TID) is based on the percentage of cortex involved by interstitial fibrosis and tubular atrophy. A semiquantitative grading of TID and interstitial inflammation was described as follows: 0=none; 1=involvement of <25%; 2=involvement of 26-50%; 3=involvement of 51-75%; 4=involvement of >76%.
Urinary HIF-1alpha levels were measured in 42 patients with LN and 30 healthy controls, using an enzyme-linked immunosorbent assay kit (Groundwork Biotechnology Diagnostics Inc., San Diego, CA, USA, Catalogue number: H108-9). All experiments were performed in duplicate.
Statistical analysis was performed using SPSS software version 11.0. Results are reported as means±SD Student's t-test was used to compare urinary HIF-1alpha levels between the LN group and the control group. Correlations between two data sets were assessed using Spearman's correlation test. p-values of <0.05 were considered significant.
Table 1 summarizes the demographic and clinical information of the 42 patients with LN. Fifteen (35.71%) patients demonstrated nephrotic range proteinuria. The most frequent histological class was WHO class IV (20/42, 47.62%). There were 8 cases with mixed classes: 5 with III plus V and 3 with IV plus V (Table 1).
Urinary HIF-1alpha levels were significantly higher in patients with LN than in healthy controls (3.977±1.696 vs. 2.153±0.554 ng/mL, p<0.001) (Fig. 1A). Urinary HIF-1alpha levels were associated with histologic CI (r=0.463, p<0.01) (Fig. 1B) and eGFR (r=-0.324, p<0.05) (Fig. 1C), but not with anti-dsDNA, ANA, C3, C4, daily proteinuria amounts or histologic AI.
LN is a severe organ manifestation of SLE and a major cause of morbidity. Whereas the immunopathogenesis of glomerular lesions in LN has been extensively studied, less is known of tubulointerstitial disease and its strong association with less favorable long-term renal prognoses.3 It has been suggested that hypoxia plays a critical role in the progression of CKD to ESRD,4,7 and tubulointerstitial hypoxia in the kidney has been considered a hallmark of injury and a mediator of disease progression.4,5 Hypoxia is a profibrogenic stimulus of tubular cells23 and changes the ECM metabolism of resident renal cells.24,25 In addition to ischemic acute renal failure, hypoxia also plays a crucial role in the development of nephrotoxic acute kidney injury, radiocontrast nephropathy, and acute glomerulonephritis.26,27
The chief mediators of this hypoxic response are hypoxia-inducible factor-1 (HIF-1) and its oxygen-sensitive component HIF-1alpha. HIF activity and the effect of HIF activation on CKD progression remain controversial. Chronic hypoxia accompanied by the up-regulation of HIF-regulated genes has pivotal roles in the progression of renal damage from ischemia/reperfusion injury,31-33 the early stage of the uninephrectomized Thy1 nephritis,34 the remnant kidney model35-37 and an obese, hypertensive type 2 diabetes rat.38
Nevertheless, long-term overactivation of HIF-1α in chronic kidney diseases is implicated to be pathogenic and is associated with the progression of chronic renal injuries.4,28-30,32,39-41 A profibrotic role of cross talk between the transforming growth factor (TGF)-β1 and HIF-1alpha signaling pathways has been reported in human renal tubular epithelial cells, as HIF-1α was shown to be necessary for hypoxic, normoxic and TGF-β1-stimulated renal cell fibrogenesis.42 Wang, et al.43 demonstrated that HIF-1α mediates angiotensin II-induced profibrotic effects through activation of cell transdifferentiation. Recently, Higgins, et al.19 also showed that hypoxia enhanced EMT of primary tubule cells through HIF-1alpha in vitro, and deletion of HIF-1alpha in tubule cells in a murine model of CKD inhibited the development of tubulointerstitial fibrosis. Kimura, et al.41 also demonstrated that stable expression of HIF-1alpha in tubular epithelial cells promoted interstitial fibrosis. HIF-1 alpha has also been reported to be up-regulated in chronic renal diseases.4,28,29,32,44 In summary, overactivation of the HIF signaling pathway might promote the progression of tubulointerstitial fibrosis, and normalization of overactivated HIF-1α under pathological conditions should antagonize excessive HIF-1alpha activity and restore normal physiological regulation. Therefore, inhibition of HIF-1alpha may be used as a treatment strategy to reduce fibrogenesis as a result of chronic kidney damage.45 In our present study, we found that urinary HIF-1alpha levels in LN patients were significantly higher than that of healthy controls, demonstrating that HIF-1alpha is increasingly expressed in the tubulointerstitial compartment vs. healthy subjects.
Our study also found the good correlations between urinary HIF-1alpha levels and the histological chronicity index eGFR, suggesting that expressed HIF-1alpha might participate in the chronicity and renal function of LN. Although the chronicity index is known to represent fibrosis, the individual components of the chronicity index indicate the degree of fibrous tissue accumulation. Considering that HIF-1alpha is expressed in renal tubular epithelial cells, our finding that urinary HIF-1alpha levels might be more reasonable to correlated with chronicity index than activity index. Thus, it would be interesting to investigate whether urinary HIF-1alpha levels can help predict renal outcome.
In summary, urinary HIF-1alpha levels were elevated in LN patients and associated with histologic chronicity changes and renal function, indicating that HIF-1alpha might contribute to histologic chronicity and renal function in LN.
The limitations of our present study include the small number of patients enrolled and lack of control for renal disease groups, as cases of different types of glomerulonephritis (i.e. primary and secondary glomerulonephritis), as well as other tubulointerstitial diseases (i.e. allograft nephropathy, drug-induced tubulointerstitial nephritis) or glomerular diseases without interstitial damage are present in all chronic renal damage cases. In addition, it remains to be proven that there is a positive correlation between urinary and renal HIF-1alpha levels and whether urinary HIF-1alpha levels accurately represent renal HIF-1alpha levels.
Figures and Tables
Fig. 1
Urinary HIF-1alpha levels in patients with lupus nephritis and in healthy controls. Urinary HIF-1alpha levels were significantly higher in patients with lupus nephritis than in healthy controls (3.977±1.696 vs. 2.153±0.554 ng/mL, p<0.001). The bars indicate group mean values (A). Urinary HIF-1alpha levels were associated with histologic chronicity indices (r=0.463, p<0.01) (B) and eGFR (r=-0.324, p<0.05) (C). HIF, hypoxia-inducible transchiption factor.

Table 1
Patients' Demographic and Clinical Information
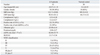
LN, lupus nephritis; SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure; ANA, antinuclear antibody; eGFR, estimated glomerular filtration rate; SLEDAI, systemic lupus erythematosis disease activity index; WHO, World Health Organization.
Values are expressed as mean±SD or number of patients (percent).
References
1. Bagavant H, Fu SM. Pathogenesis of kidney disease in systemic lupus erythematosus. Curr Opin Rheumatol. 2009. 21:489–494.

2. Yu F, Wu LH, Tan Y, Li LH, Wang CL, Wang WK, et al. Tubulointerstitial lesions of patients with lupus nephritis classified by the 2003 International Society of Nephrology and Renal Pathology Society system. Kidney Int. 2010. 77:820–829.

3. Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Tubulointerstitial damage and progression of renal failure. Kidney Int Suppl. 2005. S82–S86.

4. Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006. 17:17–25.

5. Eckardt KU, Bernhardt WM, Weidemann A, Warnecke C, Rosenberger C, Wiesener MS, et al. Role of hypoxia in the pathogenesis of renal disease. Kidney Int Suppl. 2005. S46–S51.

6. Heyman SN, Khamaisi M, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am J Nephrol. 2008. 28:998–1006.

7. Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008. 74:867–872.

8. Palm F, Friederich M, Carlsson PO, Hansell P, Teerlink T, Liss P. Reduced nitric oxide in diabetic kidneys due to increased hepatic arginine metabolism: implications for renomedullary oxygen availability. Am J Physiol Renal Physiol. 2008. 294:F30–F37.

9. Fine LG, Bandyopadhay D, Norman JT. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int Suppl. 2000. 75:S22–S26.

10. Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006. 291:F271–F281.

11. Nangaku M, Inagi R, Miyata T, Fujita T. Hypoxia and hypoxia-inducible factor in renal disease. Nephron Exp Nephrol. 2008. 110:e1–e7.

12. Henze AT, Acker T. Feedback regulators of hypoxia-inducible factors and their role in cancer biology. Cell Cycle. 2010. 9:2749–2763.

13. Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999. 15:551–578.
14. Pugh CW, Chang GW, Cockman M, Epstein AC, Gleadle JM, Maxwell PH, et al. Regulation of gene expression by oxygen levels in mammalian cells. Adv Nephrol Necker Hosp. 1999. 29:191–206.
15. Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 2001. 61:6669–6673.
16. Maxwell PH, Pugh CW, Ratcliffe PJ. Inducible operation of the erythropoietin 3' enhancer in multiple cell lines: evidence for a widespread oxygen-sensing mechanism. Proc Natl Acad Sci U S A. 1993. 90:2423–2427.

17. Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, et al. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood. 1998. 92:2260–2268.

18. Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. Hypoxic induction of Ctgf is directly mediated by Hif-1. Am J Physiol Renal Physiol. 2004. 287:F1223–F1232.
19. Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007. 117:3810–3820.

20. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997. 40:1725.

21. Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004. 65:521–530.

22. Austin HA 3rd, Muenz LR, Joyce KM, Antonovych TA, Kullick ME, Klippel JH, et al. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med. 1983. 75:382–391.
23. Manotham K, Tanaka T, Matsumoto M, Ohse T, Inagi R, Miyata T, et al. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int. 2004. 65:871–880.

24. Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000. 58:2351–2366.

25. Norman JT, Orphanides C, Garcia P, Fine LG. Hypoxia-induced changes in extracellular matrix metabolism in renal cells. Exp Nephrol. 1999. 7:463–469.

26. Kudo Y, Kakinuma Y, Iguchi M, Sato T, Sugiura T, Furihata M, et al. Modification in the von Hippel-Lindau protein is involved in the progression of experimentally induced rat glomerulonephritis. Nephron Exp Nephrol. 2007. 106:e97–e106.

27. Rosenberger C, Heyman SN, Rosen S, Shina A, Goldfarb M, Griethe W, et al. Up-regulation of HIF in experimental acute renal failure: evidence for a protective transcriptional response to hypoxia. Kidney Int. 2005. 67:531–542.

28. Nangaku M, Fujita T. Activation of the renin-angiotensin system and chronic hypoxia of the kidney. Hypertens Res. 2008. 31:175–184.

29. Higgins DF, Kimura K, Iwano M, Haase VH. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 2008. 7:1128–1132.

30. Haase VH. Pathophysiological Consequences of HIF Activation: HIF as a modulator of fibrosis. Ann N Y Acad Sci. 2009. 1177:57–65.
31. Rosenberger C, Rosen S, Shina A, Frei U, Eckardt KU, Flippin LA, et al. Activation of hypoxia-inducible factors ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrol Dial Transplant. 2008. 23:3472–3478.

32. Nangaku M, Eckardt KU. Hypoxia and the HIF system in kidney disease. J Mol Med (Berl). 2007. 85:1325–1330.

33. Hill P, Shukla D, Tran MG, Aragones J, Cook HT, Carmeliet P, et al. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2008. 19:39–46.

34. Tanaka T, Matsumoto M, Inagi R, Miyata T, Kojima I, Ohse T, et al. Induction of protective genes by cobalt ameliorates tubulointerstitial injury in the progressive Thy1 nephritis. Kidney Int. 2005. 68:2714–2725.

35. Tanaka T, Kojima I, Ohse T, Ingelfinger JR, Adler S, Fujita T, et al. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest. 2005. 85:1292–1307.

36. Song YR, You SJ, Lee YM, Chin HJ, Chae DW, Oh YK, et al. Activation of hypoxia-inducible factor attenuates renal injury in rat remnant kidney. Nephrol Dial Transplant. 2010. 25:77–85.

37. Yu X, Fang Y, Ding X, Liu H, Zhu J, Zou J, et al. Transient hypoxia-inducible factor activation in rat renal ablation and reduced fibrosis with L-mimosine. Nephrology (Carlton). 2012. 17:58–67.

38. Ohtomo S, Nangaku M, Izuhara Y, Takizawa S, Strihou CY, Miyata T. Cobalt ameliorates renal injury in an obese, hypertensive type 2 diabetes rat model. Nephrol Dial Transplant. 2008. 23:1166–1172.

39. Iwano M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens. 2004. 13:279–284.

40. Klahr S, Morrissey J. Progression of chronic renal disease. Am J Kidney Dis. 2003. 41:3 Suppl 1. S3–S7.

41. Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, et al. Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol. 2008. 295:F1023–F1029.
42. Basu RK, Hubchak S, Hayashida T, Runyan CE, Schumacker PT, Schnaper HW. Interdependence of HIF-1α and TGF-β/Smad3 signaling in normoxic and hypoxic renal epithelial cell collagen expression. Am J Physiol Renal Physiol. 2011. 300:F898–F905.

43. Wang Z, Tang L, Zhu Q, Yi F, Zhang F, Li PL, et al. Hypoxia-inducible factor-1α contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int. 2011. 79:300–310.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download