Abstract
Purpose
Coronary artery bypass grafting (CABG) is the optimal treatment option for left main coronary artery disease (LMCAD). However, LMCAD remains a constant topic of discussion between cardiac surgeons and interventional cardiologists. The aim of this study was to assess the efficacy of LMCAD treatments by comparing the mid-term outcomes of CABG and percutaneous coronary intervention (PCI) using bare metal stents or drug-eluting stents (DESs).
Materials and Methods
The study population was comprised of 199 consecutive patients admitted with unprotected LMCAD. All of the patients were assigned to PCI (88 patients) or CABG (111 patients). The primary clinical end point indicated death, stroke of acute coronary syndrome (ACS).
Results
Patients assigned to PCI were at higher operative risk than patients scheduled for CABG (6.49±4.09 vs. 4.81±2.67, p=0.0032). Comparison of the group that received DESs with the CABG group did not reveal any differences in major adverse cardio-cerebral events (MACCE) occurrence (21% vs. 16%, p=NS). Patients in the CABG and PCI groups died with similar frequency (11% vs. 16%, p=NS). The mortality rate in the CABG group was higher than among those treated with DES (11% vs. 3%, p=0.049). The rate of ACS was higher in the PCI group than in the CABG group (13% vs. 4%, p=0.016).
Left main coronary artery disease (LMCAD) incidence among patients undergoing coronary angiography is estimated to be up to 6%.1 Although uncommon, LMCAD is a constant topic of discussion between cardiac surgeons and interventional cardiologists. This discussion began in 1975, when Gorlin and Cohen2 first compared a surgical approach for LM stenosis to any other treatment options and reported that coronary artery bypass grafting (CABG) showed significant benefits over medical therapy in the treatment of LMCAD. This report began the search for alternatives to CABG in LMCAD. The first attempts with percutaneous coronary intervention (PCI) with bare metal stents (BMSs) in LM stenosis were less invasive than CABG. However, overall high mortality and revascularization rates with BMSs have led the American Heart Association and the American College of Cardiology, followed by the European Society of Cardiology, to establish firm guidelines stating that CABG is the optimal treatment option for LMCAD. By giving these pre-drug eluting stents (DESs) a class III designation, PCI was made only available to patients disqualified from all other possible methods of treatment. Since the introduction of DES, these guidelines have remained unchanged. However, as a consequence of advances in and research on stent technology, combined with the expansive development of interventional cardiology, a debate has arisen about the optimization of a medical approach to this severe coronary disease. The ability of DESs to reduce restenosis as compared to BMSs may encourage their use in LMCAD. Interventional treatment in LM stenosis has become a valuable alternative to surgery and grounds for reconsidering LMCAD treatment.
The aim of the present study was to assess the efficacy of left main coronary artery stenosis treatments by comparing the mid-term outcomes of CABG and PCI with BMSs or DESs.
The study population was comprised of 199 consecutive patients admitted between 2005 and 2008 with unprotected LMCAD. All of the patients were assigned to undergo invasive treatment; they were assigned to either PCI (88 patients) or CABG (111 patients) after a profound review of their baseline characteristics (Table 1), current standards of care in treatment for LMCAD and identification of patients at high risk using the European System for Cardiac Operative Risk Evaluation (EuroSCORE), as previously described.3 An experienced interventional cardiologist and a practiced cardiac surgeon examined each patient's case details to determine how to optimize their care. Patients who underwent PCI were those who were at a higher risk for complications from CABG, refused surgical treatment, had limited life expectancy, or were thought by the surgeon to be unsuitable for CABG.
Due to the many differences between BMSs and DESs, each of these groups was compared with the CABG group separately. For that reason, two subgroups were created: cohort 1 - CABG vs. BMS and cohort 2 - CABG vs. DES. The enrollment criterion was unprotected LMCAD occurrence, which was defined as ≥50% diameter LM stenosis on coronary angiography. The left main was considered to be unprotected when there was no coronary artery bypass graft to the left anterior descending artery (LAD) and/or the circumflex artery (Cx). Patients with concomitant valve failure requiring surgical intervention and those having already undergone bypass surgery with a graft to LAD and/or Cx were not enrolled in this study.
The diagnostic process was similar for each of the patients in the study population and included anamnesis, physical examination, basic laboratory tests, a standard 12-lead electrocardiography, echocardiographic examination and coronary angiography. The cardiac enzyme analysis was not included in the study design because they were measured only when there was clinical suspicion of ischemia.
Percutaneous coronary intervention was performed using the Seldinger technique and a transfemoral approach in all but three patients, who required a transradial approach due to peripheral vascular disease. The angiographic findings included isolated LMCAD (single left main stenosis) or LMCAD combined with one-, two- or three-vessel disease (1-VD, 2-VD, 3-VD). The stenting technique was chosen based on the location of the lesion. Ostium or trunk LM disease without bifurcation lesions were mainly treated with a single stent. Lesions involving the distal segment of the LM or bifurcation were treated with T-stenting, provisional T-stenting or the kissing-balloon technique. The operator decided between the use of a BMS or DES and chose the initial inflation pressure for high-pressure stent deployment (usually 16 atm), as well as the optimal stent apposition. Stent apposition was not evaluated by intravascular ultrasound (IVUS) because the department only became equipped with this technology in 2008. If the operator decided that the stent had been deployed incorrectly, optimal stent apposition was achieved by post-dilation with additional balloons. Percutaneous old-balloon angioplasty (POBA) was performed in patients with a history of PCI in the LM. The procedural PCI characteristics are listed in Table 3. Glycoprotein IIb/IIIa inhibitors and an intra-aortic balloon pump (IABP) were used in clinically indicated situations. The medical therapy for each patient who underwent PCI consisted of a loading dose of clopidogrel (300 or 600 mg p.o.) and unfractioned heparin (70 IU/kg i.v.). After the procedure, each patient was prescribed acetylsalicylic acid (75 mg/day) indefinitely, clopidogrel (75 mg/day) for at least twelve months, and other medications according to the judgment of the patient's physician.
The definition of procedural PCI success was a thrombolysis in myocardial infarction flow of grade 3 in the treated vessel and a final residual stenosis of <30% as well as no dissection and the absence of clinical end points.
The primary clinical end point was death of cardiac origin, stroke, and acute coronary syndrome (ACS). ACS was a composite of target vessel revascularization (TVR) and acute myocardial infarction (AMI) or unstable angina (UA) with a culprit vessel other than the LM. AMI and UA were diagnosed according to the European Society of Cardiology criteria for ACS. TVR was defined as a repeated lumen stenosis in a previously treated LM that required reintervention (within the stent, within 5 mm proximal and/or distal to the stent and within the ostium of LAD and/or Cx).
Follow-up was approximately 12 months or shorter in cases of earlier end-point occurrence. After this time, angina severity was assessed based on the Canadian Cardiovascular Society (CCS) Classification and dyspnea status according to the New York Heart Association (NYHA) Classification.
All statistical tests were performed with Statisctica software, version 9PL (StatSoft Inc., Tulsa, OK, USA) and MedCalc (MedCalc Software, Mariakerke, Belgium). Differences regarding major adverse cardio-cerebral events (MACCE), baseline and angiographic characteristics for each cohort analysis as well as differences in PCI technique between DES and BMS groups were assessed by two fractions test and a chi-square test. In order to compare length of stay, follow-up duration, EuroScore, left ventricle ejection fraction (EF), the severity of LM stenosis and stent dimentions between groups, the variables were checked for normality of distribution (Shapiro-Wilks test), and means (±SD) were calculated then compared using the nonparametric Mann-Whitney and Kruskal-Wallis tests. In order to identify independent predictors of MACCE, a multivariate logistic regression model was created with the use of baseline clinical, angiographic and procedure-related characteristics. A significance level of 0.05 was used throughout the study.
Between January 2005 and December 2008, a total of 199 patients were enrolled in this study. Of these patients, 111 were referred for CABG, 88 underwent PCI with either BMS (48 patients) or DES (34 patients), and 6 received POBA. The baseline clinical and angiographic data are presented in Table 1 and 2. Patients in the CABG group were characterized by a higher left ventricle EF of 47.9%10.2%, as compared to 41.7%13.4% in the PCI group (p<0.001). The CABG group also suffered from carotid artery disease more frequently than PCI patients (p<0.001). Compared to the CABG patients, those in the PCI group were more likely to have a history of PCI (p<0.001) and myocardial infarction as presenting symptoms (p=0.019).The average EuroSCORE was 4.81%2.67 in the CABG group and 6.49%4.09 in the PCI group (p<0.001). In 14% of surgery patients and 44% of PCI patients, a EuroSCORE of ≥ 6 was assessed.
The analysis of cohort 1 showed a significantly higher occurrence of carotid artery disease (p=0.031) and higher left ventricular ejection fraction (p<0.001) in the CABG than in the BMS group. Patients who received BMSs in the PCI procedure were older than patients in the surgery group (p=0.005). Patients receiving BMSs were also more likely to present with chronic stable angina (p<0.001) and myocardial infarction (p<0.001). The average EuroSCORE for BMS patients was 7.6%4.27 and 4.81%2.67 in the surgery group (p<0.001). A EuroSCORE of ≥6 was found in 52% of BMS patients and in 14% of CABG patients.
The comparison of the CABG and DES groups revealed that there were no cases of carotid artery disease among patients in the PCI with DES group, while 16% of CABG patients were diagnosed with this disease (p=0.007). Patients with a history of PCI were significantly more common in the DES group (p<0.001). No differences regarding clinical presentation or EuroSCORE were observed in the cohort 2 analysis. For DES patients, the mean preoperative risk was 4.76%3.36. High-risk patients (EuroSCORE ≥6) constituted 24% of the DES patients and 14% of the CABG patients.
Percutaneous interventions were performed in patients presenting with more severe LM stenosis (p=0.011). Coronary angiography revealed significant differences in the occurrence of isolated LMCAD (9% of PCI patients vs. 2% of CABG patients, p=0.008), 1-VD (28% vs. 14%, respectively, p=0.008) and 3-VD (39% vs. 60%, respectively, p=0.002). Patients also differed significantly in terms of LMCAD location. Patients with isolated ostial or mid-body disease qualified more frequently for PCI (p<0.001 and p=0.03, respectively); for those patients with stenosis in the distal bifurcation, CABG was the preferred treatment option (p<0.001).
Patients who received BMSs in the PCI procedure suffered from more severe LM stenosis than CABG patients (p<0.001). Distal-bifurcation stenosis was more frequent in the CABG group (p<0.001) and ostial left main stenosis was more frequent in the BMS group (p<0.001).
Patients receiving DES also had a higher occurrence of isolated LMCAD and 1-VD (p=0.014 and 0<0.001, respectively) as well as a higher occurrence of ostial stenosis of the LM (p<0.001). Patients undergoing CABG more frequently had 3-VD and distal-bifurcation stenosis (p<0.001 and p<0.001, respectively).
The procedural characteristics are listed in Table 3. The procedural success rate was 98% in the PCI group. Direct stenting was performed in 52 patients; 30 patients received stents after predilatation and six patients underwent POBA. The BMS subgroup consisted of 48 patients, while the DES group consisted of 34 patients. On average, 1.21±0.46 BMSs and 1.15±0.36 DESs were implanted with a total stent length of 16.88±9.35 mm for BMSs and 15.79±6.73 mm for DESs. IABP was used in 10 patients (11%), and three patients (3%) required the use of mechanical ventilation. A glycoprotein IIb/IIIa inhibitor was given to 10 patients (11%).
The outcomes are summarized in Table 4. The mean follow-up was 13.48±7.68 months for the CABG group, which was significantly different from the overall PCI group (11.44±7.21 months, p=0.038) and from the BMS group (10.56±7.46 months, p=0.002). Patients who received DES implantation were followed for 13.21±7.12 months.
In the unadjusted analysis, the combined clinical end point (MACCE) at follow-up was higher in the PCI group than in the CABG group (p=0.019). No difference in MACCE occurrence was observed between the CABG and DES patients, while significantly more patients developed MACCE after BMS implantation compared to bypass surgery (p=0.010). Individual components of the combined clinical end point revealed some heterogeniety. Patients in the CABG and PCI groups died with equal frequency. However, the mortality rate in CABG group was lower than that of BMS implantation (p=0.015) but higher than DES (p=0.049). In the CABG group the rate of ACS was lower than in the PCI group (p=0.016). There were two cases of stroke in patients after CABG and none in the PCI patients (p=0.030). TVR occurred in two patients from the DES group (6%) and in four patients in the BMS group (8%) for a total TVR of 7% in the PCI patients, compared with 4% TVR after CABG.
Multivariate logistical regression analysis was used to determine the independent MACCE-related factors and their significance. The adjusted data were method of treatment, variables listed in Table 1, 2 and 3, in-hospital length of stay and follow-up. Fig. 1 presents the outcomes.
For the overall cohort, the significant independent MACCE-related factors were diabetes mellitus and previous stroke, while EF and dyslipidemia lowered the risk of MACCE. Among patients referred to surgery, the analysis indicated peripheral vascular disease to be a significant predictor of MACCE, and EF to be a factor of freedom from MACCE.
The significant independent MACCE predictors for PCI patients were diabetes mellitus, IABP, history of stroke and age, EF, dyslipidemia and chronic stable angina decreased the probability of MACCE in those patients. In the BMS group, dyslipidemia, EF and chronic stable angina appeared to lower the end-point risk. In the DES group, diabetes mellitus and 3-VD were the independent factors significantly related to MACCE.
Post-procedural CCS Class and NYHA Class in the CABG group were similar to the scores of PCI patients at follow-up (p=0.171 for CCS and p=0.094 for NYHA). There were no differences between groups in cohort 1 and cohort 2 regarding the severity of angina or dyspnea. CABG patients stayed in the hospital longer than patients with BMS implantation (p=0.042) and also longer than patients who underwent PCI (p=0.029) (Table 4).
This study compared the mid-term outcomes of PCI and CABG in unprotected LMCAD. It showed that the higher occurrence of MACCE in the PCI group than in the CABG group (27% vs. 16%, p=0.019) may be due to the higher pre-operative risk of death in the PCI group (6.49±4.09 vs. 4.81±2.67 among CABG patients, p<0.001). Similar observations of the incidence of MACCE were also reported by the SYNTAX trial investigators (17.8% in the PCI group vs. 12.1% in the CABG group, p=0.0015).4 Some studies have found equal MACCE rates for PCI and CABG groups.5-8 However, a trend towards a higher risk of MACCE among patients who underwent PCI with DES was noticed.9 In some studies, CABG patients had a slightly higher MACCE occurrence.5,7 The outcomes of this study for PCI patients differed according to the type of stent implanted. However, the superiority of CABG over PCI in terms of MACCE occurrence suggests that the components of MACCE and subgroup analyses should be interpreted carefully. No survival benefit from BMS implantation over surgery was demonstrated, and CABG was superior to BMS in terms of mortality rates and total MACCE occurrence.
The observations comparing BMS implantation for LM stenosis with surgical revascularization are consistent with the pre-DES era studies, which suggested greater benefit from CABG than from PCI in LMCAD treatment.10 The survival rate for BMS patients in our study (73%) is in accordance with the outcomes presented in 1997 by Ellis, et al.,11 where patients diagnosed with LMCAD and disqualified from surgery were treated with BMS-supported PCI, balloon angioplasty or atherectomy. The authors reported an event-free survival of 71±5% in treated patients. Similar results were also observed a few years later.12-14 The current guidelines15 reflect those findings and state that CABG is the optimal treatment option for LM stenosis, mainly because of its well-documented and durable survival advantage.16-18 The introduction of DES to interventional practice modified the approach to LM stenosis treatment and gave solid ground reconsidering the management of this severe coronary disease.
In this study, patients who underwent PCI with DES and patients referred to CABG had comparable levels of pre-operative risk. Equal MACCE occurrence in these groups was observed, although the rate of death was lower in DES patients. Numerous previous studies comparing PCI with DES to CABG in LMCAD patients have also documented no differences in MACCE occurrence. However, the rate of TVR in patients undergoing PCI in these studies was higher than for those undergoing CABG (ARTS II, MAIN COMPARE, SYNTAX, LE MANS). Similar results for TVR occurrence were reported by Seung, et al.9 In the present study, CABG was also significantly more effective than DES implantation at reducing the need for TVR revascularization. A non-significant trend in this direction for patients with BMSs was observed. There were two cases of TVR in patients with DESs (6%) and four cases of TVR in patients with BMSs (8%), for a total TVR of 7% in PCI patients and 4% in CABG patients. For comparison, in prior registries and studies, TVR after LMCAD-stenting with DESs and BMSs ranged from 5.5% to 6% and from 11.7% to 23%, respectively.19-22 Other authors have reported rates of TVR in PCI with DES and CABG groups at 6-14 months follow-up similar to the present study.5-7,23 The higher rate of TVR with PCI compared to CABG appeared to translate into a significant increase in the rate of death, but only in BMS patients. The higher rate of TVR in PCI with DES patients compared to CABG patients was not reflected in an overall increase in death or myocardial infarction. Moreover, patients died more frequently after CABG. The risk of TVR in PCI should be balanced against the invasiveness of surgical procedures and the risk of stroke related to CABG (2% vs. 0% in the PCI group, p=0.03 in current study), which has been found by numerous previous studies to be more common in this group.4,7 The lower rate of stroke after PCI may be due to the more frequent use of dual antiplatelet therapy after stent implantation than after surgery, reflecting the differences in current guidelines and standard care between the two groups.24 The use of thienopyridine (ticlopidine or clopidogrel) at follow-up reached 64% in PCI patients, while only 10% of patients received such treatment after CABG. A comparable rate of patients receiving thienopyridine one year after PCI was reported in the SYNTAX Trial (71.1%).4 However, the influence of drug therapy on reducing stroke occurrence is just a hypothesis and was not proven by any statistical method. Based on multivariate logistic-regression analysis, independent stroke, death and ACS (MACCE)-related factors and their levels of significance are presented in Table 4. The analysis conducted on the general cohort indicated that diabetes mellitus and previous stroke were strong independent predictors of MACCE, while left ventricle ejection fraction and dyslipidemia appeared to lower the risk of MACCE. The controversial finding concerning dyslipidemia may be related to previous chronic drug treatment for this disease (for example, a pleiotropic statin effect), and its potentially positive effect on the outcome and decrease in MACCE risk. The influence of diabetes mellitus on MACCE occurrence for the general cohort is in accordance with previous papers.7,24 The analysis did not indicate any method of treatment (CABG or PCI) to be a predictor of MACCE. The logistic regression model was also applied to each of the subgroups separately. Diabetes mellitus appeared to be one of the predictors of MACCE in the analysis of PCI, DES and BMS patients, while it was not a significant factor for MACCE in the CABG group. These findings are not consistent with the results of the Bypass Angioplasty Revascularization Investigation trial,25 in which the 7-year mortality rate of treated diabetics in the registry was equal in the PCI and CABG groups. Notwithstanding those observations, according to the clinical recommendations, the preferred treatment option for patients presenting with chronic stable angina and multivessel CAD or LMCAD-especially with concomitant diabetes mellitus-is surgical revascularization.26
It should be noted that interpretation of the complex MACCE etiology based solely on logistic regression outcomes is incomplete. The complexity of the patient's clinical status and the procedural characteristics should be included in the analysis as operator- and guideline-dependent factors.
In order to identify patients at high risk and predict the risk of mortality, we used the EuroSCORE. Patients in the PCI group were revealed to be at higher pre-operative risk than patients referred to surgery. The comparison of EuroSCOREs between CABG and DES patients showed equal scores in the two groups. Notwithstanding the recommendations for high-risk patients who ought to be treated surgically, there is still a divergence in the methods applied and outcomes reported by many different investigators. In several prior studies, according to the recommendations, patients with more significant comorbidities were scheduled for CABG;21,27 however, some other authors have reported a tendency to treat such patients with PCI.5,28 Death and myocardial infarction rates seemed to be noninferior among PCI patients at lower perioperative risk in a randomized prospective trial comparing percutaneous (sirolimus eluting stents) and surgical methods of treatment for unprotected LMCAD.23
LM stenosis is potentially an attractive target for interventional treatment because it is the most proximal segment of the left coronary artery and is usually a vessel of large diameter. However, an isolated LMCAD is a rare angiographic finding (6-9% of LM stenoses). Furthermore, the predilection of LMCAD for locations in the distal bifurcation makes PCI a challenge for interventional cardiologists, regardless of the type of device and the technique used. Moreover, 70-80% of patients have concomitant 3-VD5-7,19,29-32 and, combined with the relative frequency of distal bifurcation lesions (53-90% of patients) extending into the proximal coronary arteries (Cx, LAD),7,30,31,33 may favor CABG over PCI for LM stenosis to enable more complete surgical revascularization. In the present study, 50% of the patients with LMCAD suffered from 3-VD (39% in the PCI group vs. 60% in the CABG group, p=0.002), and, in 75% of cases, the lesion was located in the distal LM (59% in PCI group vs. 87% in CABG group, p<0.001). Several authors have observed a higher incidence of TVR at the ostium of the circumflex artery after stent implantation in the distal bifurcation.5,19,30,34 The crucial importance of distal LM stenosis as a predictor of adverse outcomes was emphasized by Valgimigli, et al.20 These study results do not agree with those mentioned above and do not allow for the conclusion that there is a direct relationship between stenosis location and MACCE occurrence. Distal bifurcation patients were assigned by convenience treatment and more frequently underwent CABG. This assignment did not influence MACCE occurrence in this group, which was statistically less frequent than among PCI patients.
Although there were differences in MACCE occurrence, the clinical status of patients after PCI and CABG did not differ significantly. Post-procedural angina and dyspnea status assessed at follow-up did not differ between the groups in the general cohort analysis, a finding in accordance with previous studies.21,23 CABG patients had rates of angina and dyspnea comparable to those of DES and BMS patients.
The main limitations of our study are the relatively small number of registered patients; lack of randomization; mixed-group, one-center experience; retrospective analysis and lack of IVUS guided procedures. As in many similar studies, it is also limited by the selective performance of PCI in patients considered to be unsuitable for CABG.
Despite the fact that patients treated with PCI were at higher operative risk, PCI with DES for LMCAD was comparable to CABG in terms of mortality, ACS and stroke (MACCE) at midterm follow-up. PCI patients had a significantly higher risk of TVR. PCI with DES is safe and could represent a good alternative to CABG for selected cases of patients with unprotected LMCAD.
Figures and Tables
Fig. 1
Outcomes. DES, drug-eluting stent; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; BMS, bare metal stents; CI, confidence interval; EF, ejection fraction; IABP, intra-aortic balloon pump; LM+3VD, left main combined with three-vessel disease; MACCE, major adverse cardio-cerebral events; OR, odds ratio.

Table 3
Procedural Characteristics
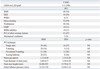
BMS, bare metal stent; CABG, coronary artery bypass grafting; DES, drug-eluting stent; IABP, intra-aortic balloon pump; LAD, left anterior descending; LIMA, left internal mammary artery; PCI, percutaneous coronary intervention; POBA, percutaneous old-balloon angiography; NS, not significant.
Values are n (%) or mean±SD.
References
1. Stone GW, Moses JW, Leon MB. Left main drug-eluting stents: natural progression or a bridge too far? J Am Coll Cardiol. 2007. 50:498–500.
2. Cohen MV, Gorlin R. Main left coronary artery disease. Clinical experience from 1964-1974. Circulation. 1975. 52:275–285.

3. Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. 1999. 16:9–13.

4. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009. 360:961–972.

5. Lee MS, Kapoor N, Jamal F, Czer L, Aragon J, Forrester J, et al. Comparison of coronary artery bypass surgery with percutaneous coronary intervention with drug-eluting stents for unprotected left main coronary artery disease. J Am Coll Cardiol. 2006. 47:864–870.

6. Palmerini T, Marzocchi A, Marrozzini C, Ortolani P, Saia F, Savini C, et al. Comparison between coronary angioplasty and coronary artery bypass surgery for the treatment of unprotected left main coronary artery stenosis (the Bologna Registry). Am J Cardiol. 2006. 98:54–59.

7. Chieffo A, Morici N, Maisano F, Bonizzoni E, Cosgrave J, Montorfano M, et al. Percutaneous treatment with drug-eluting stent implantation versus bypass surgery for unprotected left main stenosis: a single-center experience. Circulation. 2006. 113:2542–2547.

8. Chieffo A, Park SJ, Valgimigli M, Kim YH, Daemen J, Sheiban I, et al. Favorable long-term outcome after drug-eluting stent implantation in nonbifurcation lesions that involve unprotected left main coronary artery: a multicenter registry. Circulation. 2007. 116:158–162.

9. Seung KB, Park DW, Kim YH, Lee SW, Lee CW, Hong MK, et al. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008. 358:1781–1792.

10. Dzavik V, Ghali WA, Norris C, Mitchell LB, Koshal A, Saunders LD, et al. Long-term survival in 11,661 patients with multivessel coronary artery disease in the era of stenting: a report from the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) Investigators. Am Heart J. 2001. 142:119–126.

11. Ellis SG, Tamai H, Nobuyoshi M, Kosuga K, Colombo A, Holmes DR, et al. Contemporary percutaneous treatment of unprotected left main coronary stenoses: initial results from a multicenter registry analysis 1994-1996. Circulation. 1997. 96:3867–3872.

12. Tan WA, Tamai H, Park SJ, Plokker HW, Nobuyoshi M, Suzuki T, et al. Long-term clinical outcomes after unprotected left main trunk percutaneous revascularization in 279 patients. Circulation. 2001. 104:1609–1614.

13. Kelley MP, Klugherz BD, Hashemi SM, Meneveau NF, Johnston JM, Matthai WH Jr, et al. One-year clinical outcomes of protected and unprotected left main coronary artery stenting. Eur Heart J. 2003. 24:1554–1559.

14. Lee BK, Hong MK, Lee CW, Choi BR, Kim MJ, Park KH, et al. Five-year outcomes after stenting of unprotected left main coronary artery stenosis in patients with normal left ventricular function. Int J Cardiol. 2007. 115:208–213.

15. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, Jacobs AK, Kern MJ, King SB 3rd, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J Am Coll Cardiol. 2006. 47:e1–e121.
16. Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994. 344:563–570.

17. Caracciolo EA, Davis KB, Sopko G, Kaiser GC, Corley SD, Schaff H, et al. Comparison of surgical and medical group survival in patients with left main equivalent coronary artery disease. Long-term CASS experience. Circulation. 1995. 91:2335–2344.

18. Eagle KA, Guyton RA, Davidoff R, Edwards FH, Ewy GA, Gardner TJ, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: summary article A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). J Am Coll Cardiol. 2004. 44:e213–e310.
19. Valgimigli M, van Mieghem CA, Ong AT, Aoki J, Granillo GA, McFadden EP, et al. Short- and long-term clinical outcome after drug-eluting stent implantation for the percutaneous treatment of left main coronary artery disease: insights from the Rapamycin-Eluting and Taxus Stent Evaluated At Rotterdam Cardiology Hospital registries (RESEARCH and T-SEARCH). Circulation. 2005. 111:1383–1389.

20. Valgimigli M, Malagutti P, Rodriguez Granillo GA, Tsuchida K, Garcia-Garcia HM, van Mieghem CA, et al. Single-vessel versus bifurcation stenting for the treatment of distal left main coronary artery disease in the drug-eluting stenting era. Clinical and angiographic insights into the Rapamycin-Eluting Stent Evaluated at Rotterdam Cardiology Hospital (RESEARCH) and Taxus-Stent Evaluated at Rotterdam Cardiology Hospital (T-SEARCH) registries. Am Heart J. 2006. 152:896–902.

21. Buszman PE, Kiesz SR, Bochenek A, Peszek-Przybyla E, Szkrobka I, Debinski M, et al. Acute and late outcomes of unprotected left main stenting in comparison with surgical revascularization. J Am Coll Cardiol. 2008. 51:538–545.

22. Erglis A, Narbute I, Kumsars I, Jegere S, Mintale I, Zakke I, et al. A randomized comparison of paclitaxel-eluting stents versus bare-metal stents for treatment of unprotected left main coronary artery stenosis. J Am Coll Cardiol. 2007. 50:491–497.

23. Boudriot E, Thiele H, Walther T, Liebetrau C, Boeckstegers P, Pohl T, et al. Randomized comparison of percutaneous coronary intervention with sirolimus-eluting stents versus coronary artery bypass grafting in unprotected left main stem stenosis. J Am Coll Cardiol. 2011. 57:538–545.

24. King SB 3rd, Smith SC Jr, Hirshfeld JW Jr, Jacobs AK, Morrison DA, Williams DO, et al. 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee. Circulation. 2008. 117:261–295.

25. Feit F, Brooks MM, Sopko G, Keller NM, Rosen A, Krone R, et al. Long-term clinical outcome in the Bypass Angioplasty Revascularization Investigation Registry: comparison with the randomized trial. BARI Investigators. Circulation. 2000. 101:2795–2802.

26. Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, et al. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006. 27:1341–1381.

27. Dudek D, Heba G, Giszterowicz D, Rzeszutko Ł, Legutko J, Bartuś S, et al. Stenting of unprotected left main coronary artery in patients with low preoperative risk of coronary artery bypass grafting. Kardiol Pol. 2006. 64:929–936.
28. Brener SJ, Galla JM, Bryant R 3rd, Sabik JF 3rd, Ellis SG. Comparison of percutaneous versus surgical revascularization of severe unprotected left main coronary stenosis in matched patients. Am J Cardiol. 2008. 101:169–172.

29. de Lezo JS, Medina A, Pan M, Delgado A, Segura J, Pavlovic D, et al. Rapamycin-eluting stents for the treatment of unprotected left main coronary disease. Am Heart J. 2004. 148:481–485.

30. Park SJ, Kim YH, Lee BK, Lee SW, Lee CW, Hong MK, et al. Sirolimus-eluting stent implantation for unprotected left main coronary artery stenosis: comparison with bare metal stent implantation. J Am Coll Cardiol. 2005. 45:351–356.

31. Price MJ, Cristea E, Sawhney N, Kao JA, Moses JW, Leon MB, et al. Serial angiographic follow-up of sirolimus-eluting stents for unprotected left main coronary artery revascularization. J Am Coll Cardiol. 2006. 47:871–877.

32. Kim YH, Park SW, Hong MK, Park DW, Park KM, Lee BK, et al. Comparison of simple and complex stenting techniques in the treatment of unprotected left main coronary artery bifurcation stenosis. Am J Cardiol. 2006. 97:1597–1601.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download