Abstract
Purpose
The aim of this study was to evaluate the recent changes in the clinicopathologic features of prostate cancer in Korea and to compare these features with those of Western populations.
Materials and Methods
We retrospectively reviewed the data of 1582 men undergoing radical prostatectomy for clinically localized prostate cancer between 1995 and 2007 at 10 institutions in Korea for comparison with Western studies. The patients were divided into two groups in order to evaluate the recent clinicopathological changes in prostate cancer: Group 1 had surgery between 1995 and 2003 (n=280) and Group 2 had surgery between 2004 and 2007 (n=1302). The mean follow-up period was 24 months.
Results
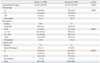
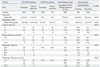
Group 1 had a higher prostate-specific antigen level than Group 2 (10.0 ng/mL vs. 7.5 ng/mL, respectively; p<0.001) and a lower proportion of biopsy Gleason scores ≤6 (35.0% vs. 48.1%, respectively; p<0.001). The proportion of patients with clinical T1 stage was higher in Group 2 than in Group 1. Group 1 had a lower proportion of organ-confined disease (59.6% vs. 68.6%; p<0.001) and a lower proportion of Gleason scores ≤6 (21.3% vs. 33.0%; p<0.001), compared to Group 2. However, the relatively higher proportion of pathologic Gleason scores ≤6 in Group 2 was still lower than those of Western men, even though the proportion of organ-confined disease reached to that of Western series.
Prostate cancer is known to be the most common male cancer and the second most common cause of cancer-related mortality in the Unites States.1,2 Several factors have been reported to be risk factors for prostate cancer. These factors include aging, race, and high-fat diets.1 Racial differences have been reported to impact the incidence and behavior of prostate cancer.1,2
Korea is one of the most rapidly aging countries in the world and the proportion of the population ≥65 years of age has increased from 5.1% in 1990 to 9.1% in 2005.3,4 Accordingly, the number of patients with prostate-related disease is increasing rapidly.
The incidence of prostate cancer in 2007 was fifth among all malignancies in Korean men.5 Although its incidence in Korea is relatively lower than that of Western countries, e.g. United States (155.5 per 100000 person-years), the age adjusted incidence rates of prostate cancer in Korean according to the Korean Cancer Registry System rapidly increased from 10.1 per 100000 person-years in 2002 to 20.0 per 100000 person-years in 2007.2,5,6 The incidence of prostate cancer in Korea is increasing due to several reasons including public awareness programs and life styles change.7 Interestingly, recent data has shown poor differentiation of prostate cancers in Korean men.8 However, it is not clear whether ethnic differences truly affect the clinicopathological features of this cancer in Korea.
This study was designed to evaluate the recent changes in clinicopathological features of prostate cancer in Korean men and to compare these presenting features with Western studies.
We retrospectively reviewed data from 1582 men who had undergone radical prostatectomy for clinically localized prostate cancer between 1995 and 2007 at 10 institutions in Korea. This study was approved by the Institutional Review Board of Samsung Medical Center. Patients who had received hormonal therapy or radiotherapy before radical prostatectomy or those without sufficient pre-operative clinical or pathologic data were excluded from the analysis. Needle biopsy specimens were taken from 6 (10.9%), 8 (8.4%), 10 (14.8%), and 12 cores (65.8%), and additional cores if suspicious lesions were detected therein. The pathologic data were reviewed again by a group of expert uropathologists. All of the pathology slides from the 18-gauge needle biopsy specimens, as well as surgical prostate specimens, were re-evaluated at participating medical centers. The clinical and pathologic stage was assigned according to the 2002 tumor-node-metastasis staging system, with histologic grading determined in accordance with the Gleason grading system.9 Beginning 6-8 weeks after radical prostatectomy, the prostate-specific antigen (PSA) level was measured every 3-6 months. Biochemical failure was defined as having a PSA concentration ≥0.2 ng/mL on two consecutive occasions after having achieved an undetectable PSA level.10
The incidence of prostate cancer in Korea is increasing, and the number of radical prostatectomies has increased, correspondingly. Therefore, patients were divided into the following two groups in order to evaluate the recent changes in characteristics of prostate cancer in Korea: Group 1 had surgery between 1995 and 2003 (n=280) and Group 2 had surgery between 2004 and 2007 (n=1302). The separation of the two groups was based on a previous study involving the characteristics of prostate cancer in Korea.8 The pre-operative clinicopathological parameters and post-operative variables, such as age, PSA, clinical stage, biopsy Gleason score, pathologic stage, and final Gleason score were compared between the two groups. The mean follow-up period was 24 months (range, 1-98 months). The 2-year biochemical recurrence-free survival was compared between the two groups due to the relatively short follow-up period in Group 2.
We chose the Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE) databases, Shared Equal Access Regional Cancer Hospital (SEARCH) databases and a large retrospective Western study to compare the clinicopathological features of prostate cancer patients undergoing radical prostatectomy in Korean and Western countries.11-13
Using the Statistical Package for Social Sciences (SPSS) software, version 17.0 (SPSS Inc., Chicago, IL, USA), we tested the distribution of clinicopathologic parameters using chi-square and Student t-tests. A Kaplan-Meier survival curve was obtained and biochemical recurrence-free survival was compared using a log rank test. A p-value <0.05 was set for determination of significance.
The final study comprised 1582 men (mean age, 65.0 years; range, 38-85 years) with a median pre-operative serum PSA level of 9.0 ng/mL (range, 0.2-233.9 ng/mL) and a median biopsy Gleason score of 7. The pre-operative clinicopathological characteristics are shown in Table 1 according to each group. Group 2 (surgery between 2004 and 2007) was comprised of older patients (p=0.002). The proportion of patients with clinical T1 stage was higher in Group 2 than in Group 1 and the proportion of low Gleason scores (≤6) was correspondingly higher in Group 2 (p=0.003 and p<0.001, respectively). The median PSA level decreased from 10.0 ng/mL in Group 1 to 7.5 ng/mL in Group 2. Patients with a PSA level <10.0 ng/mL totaled 50.0% and 66.1% of Group 1 and Group 2, respectively (p<0.001 by chi-square test).
Pathologic data demonstrated that organ-confined disease increased from 59.6% in Group 1 to 68.6% in Group 2 (p<0.001 by chi-square test) (Table 2). Patients with moderately and poorly differentiated cancers (Gleason score ≥7) accounted for 78.7% and 67.0% of Group 1 and Group 2, respectively (p<0.001 by chi-square test). Group 2 presented better characteristics in terms of pathologic stage and histologic differentiation compared to Group 1 (Table 2).
Survival analysis showed that the biochemical-recurrence free survival favored Group 2 (p<0.001, by log-rank test) (Fig. 1). The calculated actuarial biochemical failure-free survival rate at 2 years was 61.3% and 86.1% for Group 1 and Group 2, respectively.
We compared our results with the clinicopathological parameters of Westerners collected in large-scale series including the Prostate Strategic Urological Research Endeavor (CaPSURE) databases, SEARCH databases and a large retrospective Western study.11-13 Western series showed younger age and lower PSA levels compared to the groups in our study (p<0.001). Also, biopsy Gleason scores in Western series showed much higher proportions of patients with Gleason score of 6 than Korean counterparts (p<0.001). Distribution of final pathologic stages was similar between patients in Group 2 of the present study and the SEARCH database (Caucasians and African Americans, p=0.08 and p=0.88, respectively). However, Koreans demonstrated a higher proportion of worse pathologic Gleason scores (Gleason score ≥7, p<0.001) (Table 3).
Several factors have been reported to serve as risk factors for prostate cancer; specifically, aging, family history, and a animal-protein rich diet are known to increase the risk of prostate cancer.1 African-American men have a tendency to contract this disease more often compared to Caucasian men; however, studies on ethnic differences as they relate to prostate cancer in Asians are scarce.13,14 The incidence of prostate cancer is most common in western countries where Asians comprise a lower proportion of the general population. It is well-known that immigrant men from Japan in the United States have a higher incidence of prostate cancer compared to their counterparts in their native country.15,16 Therefore, some environmental factors, such as a westernized diet (low in vegetables and high in fat and protein), may explain the higher incidence in these immigrant men. Some Asian countries have rapidly developed and lifestyles have been westernized. Korea is one such country and the incidence of prostate cancer is increasing rapidly. As aforementioned, prostate cancer was the fifth most common cancer in Korea in 2007 and the most rapidly increasing cancer in Korean men according to the Korean Cancer Registry.5 Each year, the Korean Urological Association releases data on the number of radical prostatectomies performed in resident-training centers in Korea. The number of prostatectomies was reported to increase from 348 in 2002 to 2,123 in 2007.17 The basis for this increase in incidence of prostate cancer included increased longevity, westernized lifestyle, easier access to medical facilities for the general population, and the launch of several public awareness programs.7,18 Because of this situation, a number of urologists are interested in the behavior of prostate cancer in Korean men compared to other ethnic groups. However, few studies have reported the role of ethnic differences in Asians in terms of risk factors and characteristics of prostate cancer. This study was performed to evaluate the characteristics of prostate cancer in Korean men and to compare the presenting features of prostate cancer in Korea with those of Western studies.
African-American patients have been reported to experience worse outcomes compared to Caucasian counterparts.14 Cohen, et al.19 reported a shorter disease-free survival in 2814 African-American men compared to other ethnicities in their analysis of the Surveillance, Epidemiology, and End Results Program-Medicare database, including 23353 Caucasian, 480 Hispanic, and 566 Asian men. The latter three ethnic groups demonstrated no statistical differences in disease-free survival. However, Song, et al.8 reported that prostate cancer in 604 Korean men exhibited poor differentiation and was adversely related to prognosis after radical prostatectomy. A relatively higher proportion of high-grade cancers, irrespective of initial serum PSA concentrations or the clinical stage, may be responsible for the greater serum PSA level at the time of presentation and eventually result in a greater rate of PSA failure, even in those with pathologic organ-confined disease. They suggested poorer outcomes for prostate cancer in Korean men via a plausible mechanism involving an increased incidence of prostate cancer and the lower serum testosterone levels in Asians.8,20-22 However, the relevance of serum testosterone level and profiles of prostate cancer is still controversial.23
In the present study, Group 2 had more organ-confined disease and a favorable histologic differentiation. These two features are important variables to predict outcomes of prostate cancer. They provided Group 2 with improved short-term biochemical recurrence free survival. However, the relatively higher proportions of pathologic Gleason scores ≤6 (33.0%) in Group 2 is still lower compared to western men (40.0-61.1%), even though the proportion of organ-confined disease reached to that of their western counterparts.24-26 Notwithstanding, Korean men still present poorer outcomes because of relatively worse pathologic parameters compared to western men. The exact mechanisms underlying poorer pathologic Gleason scores in the recently managed Korean men (Group 2) cannot be determined due to the limited data collected in this retrospective study.
Comparing Group 1 with Group 2, we wondered what led to the better profiles of clinical and pathologic parameters in Group 2. First, Koreans are becoming more knowledgeable on prostate cancer through public awareness programs and an increased interest in health issues.18 Accordingly, a greater number of men visit hospitals to determine their prostate status even though they have no symptoms. Many Koreans perform health check-ups to identify underlying health problems and these generally cover PSA screening in Korean men. This has led to earlier detection of prostate cancer in asymptomatic men. Second, the recent rapid increase in prostate cancer has piqued physicians' interest in this disease and more physicians have begun testing PSA in their daily clinical activities.
We expected to find poorer biochemical recurrence free survival compared to Western populations. And it was not easy to understand this difference completely through retrospective series, several factors, including ethnic and environmental differences, may have had an impact on our results. First of all, we should consider the relatively short follow-up period of Group 2 of our study as one limitation in our study. We would need a longer follow-up in order to compare long-term Korean biochemical recurrence free and cancer-specific survivals with that those of Western populations.
There were several other limitations in this study. The retrospective nature of this study may have created a selection bias, which in turn could lead to interference with accurate analysis of the characteristics of prostate cancer. The socioeconomic factors such as education and income could affect differences in disease progression among Asians, Caucasians and African Americans, but the lack of such data limited our evaluation thereof. The lack of central pathologic review was also a limitation in our study.
In conclusion, Korean men with prostate cancer currently present better clinical and pathologic parameters than men in Western countries. However, the pathologic Gleason score of Korean men remained poor, which might be attributable to ethnic differences in the behavior of prostate cancer.
Figures and Tables
ACKNOWLEDGEMENTS
This research was supported by a Korean Urological Oncology Society Grant 08-01 sponsored by Korean Ipsen.
References
1. Giovannucci E, Platz EA. Vogelzang NJ, Scardino PT, Shipley WU, Debruyne FMJ, Linehan WM, editors. Epidemiology of prostate cancer. Comprehensive Textbook of Genitourinary Oncology. 2006. 3rd ed. Philadelphia: Lippincott Williams & Wilkins;9–22.
3. Korea Statistical Information Service. accessed on 2010 October 30. Available at: http://www.kostat.go.kr.
4. Kim KI, Chang HJ, Cho YS, Youn TJ, Chung WY, Chae IH, et al. Current status and characteristics of hypertension control in community resident elderly Korean people: data from a Korean longitudinal study on health and aging (KLoSHa study). Hypertens Res. 2008. 31:97–105.

5. http://www.ncc.re.kr. Accessed March 2012.
6. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.

7. Seo HK, Chung MK, Ryu SB, Lee KH. Korean Urological Oncologic Society Prostate Cancer Study Group. Detection rate of prostate cancer according to prostate-specific antigen and digital rectal examination in Korean men: a nationwide multicenter study. Urology. 2007. 70:1109–1112.

8. Song C, Ro JY, Lee MS, Hong SJ, Chung BH, Choi HY, et al. Prostate cancer in Korean men exhibits poor differentiation and is adversely related to prognosis after radical prostatectomy. Urology. 2006. 68:820–824.

9. Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL. ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005. 29:1228–1242.

10. Freedland SJ, Sutter ME, Dorey F, Aronson WJ. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology. 2003. 61:365–369.

11. Grossfeld GD, Latini DM, Downs T, Lubeck DP, Mehta SS, Carroll PR. Is ethnicity an independent predictor of prostate cancer recurrence after radical prostatectomy? J Urol. 2002. 168:2510–2515.

12. Freedland SJ, Amling CL, Dorey F, Kane CJ, Presti JC Jr, Terris MK, et al. Race as an outcome predictor after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Urology. 2002. 60:670–674.

13. Hernandez DJ, Nielsen ME, Han M, Partin AW. Contemporary evaluation of the D'amico risk classification of prostate cancer. Urology. 2007. 70:931–935.

14. Godley PA, Schenck AP, Amamoo MA, Schoenbach VJ, Peacock S, Manning M, et al. Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. J Natl Cancer Inst. 2003. 95:1702–1710.

15. Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968. 40:43–68.
16. Yu H, Harris RE, Gao YT, Gao R, Wynder EL. Comparative epidemiology of cancers of the colon, rectum, prostate and breast in Shanghai, China versus the United States. Int J Epidemiol. 1991. 20:76–81.

17. The Korean Urological Association. Data on statistics of management in resident training hospital in the Korean Urological Association in the year 2007. Korean J Urol. 2008. 49:1171–1172.
18. Song C, Ahn H, Lee MS, Park J, Kwon TG, Kim HJ, et al. Mass screening for prostate cancer in Korea: a population based study. J Urol. 2008. 180:1949–1952.

19. Cohen JH, Schoenbach VJ, Kaufman JS, Talcott JA, Schenck AP, Peacock S, et al. Racial differences in clinical progression among Medicare recipients after treatment for localized prostate cancer (United States). Cancer Causes Control. 2006. 17:803–811.

20. van Houten ME, Gooren LJ. Differences in reproductive endocrinology between Asian men and Caucasian men--a literature review. Asian J Androl. 2000. 2:13–20.
21. Wu AH, Whittemore AS, Kolonel LN, Stanczyk FZ, John EM, Gallagher RP, et al. Lifestyle determinants of 5alpha-reductase metabolites in older African-American, white, and Asian-American men. Cancer Epidemiol Biomarkers Prev. 2001. 10:533–538.
22. Hoffman MA, DeWolf WC, Morgentaler A. Is low serum free testosterone a marker for high grade prostate cancer? J Urol. 2000. 163:824–827.

23. Hsing AW, Chu LW, Stanczyk FZ. Androgen and prostate cancer: is the hypothesis dead? Cancer Epidemiol Biomarkers Prev. 2008. 17:2525–2530.

24. Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004. 172:910–914.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download