Abstract
Purpose
Some recent trials suggest that postoperative adjuvant radiotherapy (RT) may be safely omitted after breast-conserving surgery (BCS) for some patients with ductal carcinoma in situ (DCIS). In this study, we reviewed clinical outcomes of patients with DCIS treated with partial mastectomy (PM) without adjuvant RT.
Materials and Methods
Medical records of 28 patients (29 breasts) with DCIS who were treated with PM, but without RT, between April 1991 and December 2010 were retrospectively analyzed. Based on established criteria (2.0 cm or less in size and no comedonecrosis), 18 patients were treated without RT after PM. Seven patients (8 breasts) who did not receive RT due to refusal were also included in this study. Three other patients were excluded because data concerning comedonecrosis were not available.
Results
For the 25 patients included in this study, the mean age of the 18 patients who met the criteria was 47.9±6.2 years, and 47.6±12.7 years for the 7 patients who did not. The mean sizes of the primary tumors were 0.6±0.4 cm and 0.9±0.3 cm, respectively, in these two groups. Among these 25 patients (26 breasts) treated without RT, we observed no ipsilateral breast tumor recurrence or mortality within a mean follow-up of 84 months.
Ductal carcinoma in situ (DCIS) is a noninvasive disease with heterogeneous histologic features confined to the ductal lumen of the breast. With increasing use of screening mammography and technical advances in diagnostic radiology, the diagnosis of DCIS has dramatically increased. Indeed, the incidence of DCIS in Korea has increased in the past 10 years, from 4.2% in 1996 to 9.6% in 2006, as compared to 20-25% in the United States.1,2
In the early 1980s, most patients with DCIS were treated with mastectomy, which resulted in very low rates (1% to 2%) of local recurrence.3 Since then, however, four large randomized controlled trials (RCTs) reported comparable and acceptably low rates of local recurrence for patients with DCIS who were treated with breast-conserving surgery (BCS) followed by radiation therapy (RT). Today, BCS with RT is considered as a standard treatment for patients with DCIS,4-9 and in the United States, it is now the most common treatment for this form of breast cancer.10
In two retrospective studies, however, patients with small lesions that had been widely excised who did not receive subsequent adjuvant RT had outcomes comparable to those of patients who did receive RT; patients with tumors lacking high-grade features did especially well among those for whom RT was omitted.11,12 Although a meta-analysis of the previously cited RCTs revealed an approximate 60% reduction in breast cancer recurrence with the addition of radiation, the additional treatment did not extend survival or reduce distant metastases as compared to excision alone. Moreover, adjuvant RT was associated with a 1.53-fold increase in risk for contralateral breast cancer.13 In this setting, the use of adjuvant RT presents a dilemma. If survival cannot be extended, prevention of local recurrence becomes the major treatment goal. Although RT may reduce local recurrence, it increases morbidity and inconvenience.14 Accordingly, there is intensive debate concerning whether to omit adjuvant RT for some patients with DCIS.
In this retrospective study, at our single institution, we investigated the long-term outcomes of patients with DCIS treated with partial mastectomy (PM) without adjuvant RT.
Patients diagnosed with DCIS and treated with PM without RT between April 1991 and December 2010 at the Gangnam Severance Hospital in Korea were identified, and data were extracted from their records retrospectively. The internal review board at this institution waived the informed consent requirement for this retrospective study.
Among the 2933 patients (3062 breasts) diagnosed in this period, 302 patients (10.3%) with DCIS were treated. Of these patients, 87 (28.8%) were treated with PM. Fifty-one patients (58.6%) were treated with RT, and 28 patients (32.2%) were treated without RT. Eight patients (9.2%) were excluded from this study because data regarding RT were not available. Eligibility criteria for omission of adjuvant RT after PM were 1) DCIS lesions measuring ≤2.0 cm; and 2) absence of comedonecrosis. Each patient was informed of her own pathologic status and of the option to omit RT from the standard regimen for DCIS. The patient was then asked to decide whether adjuvant RT should be implemented, in accordance with her preference. Of the 28 patients for whom RT was omitted, 8 patients strongly preferred the omission, in spite of the presence of comedonecrosis.
In all patients, partial mastectomy was performed with at least a 2-cm macroscopic tumor-free margin, and the negative resection margin status was defined as 'tumor not touching ink' on the frozen section. The size of the DCIS lesion was determined from pathology reports and medical records. When both data were unavailable, the mammographic abnormality was measured on the preoperative mammogram.
Adjuvant hormonal therapy was routinely employed for patients who had positive estrogen and/or progesterone receptor status. After treatment, patients were monitored on a regular basis.
Statistical calculations were performed with SAS (version 9.1, Cary, NC, USA). The χ2 test or Fisher's exact test, and the t-test were used to evaluate relationships between clinicopathologic variables and comedonecrosis. The recurrence and contralateral breast event (CBE) rates were determined by the Kaplan-Meier method based on the survival data. Survival curves were compared using the log-rank test. p values <0.05 were considered statistically significant.
Table 1 lists clinicopathologic characteristics of patients treated with PM without adjuvant RT.
For 18 of 25 patients who met target criteria in this study, the mean follow-up period was 85.2±60.5 months; and for 7 patients (8 breasts) who did not meet the criteria, follow-up was 81±44.4 months (p=0.863). Mean ages in the two groups were 47.9±6.2 years and 47.6±12.7 years (p=0.948), respectively, and mean sizes of the primary tumor were 0.6±0.4 cm and 0.9±0.3 cm (p=0.048), respectively. In the Van Nuys prognostic classification, 15 (83.3%) of the 18 patients who met the criteria were group 1. Among 7 patients who did not meet the criteria, none was group 1, while 6 patients (75.0%) were group 2 and one (12.5%) was group 3 (p<0.001). In none of the 25 patients in the study were multifocal tumors observed.
One patient had a positive resection margin on her permanent evaluation and radiation was omitted due to patient refusal, although all margins were negative on frozen section. Adjuvant hormone therapy was administered to 13 patients (76.5%).
Patients with invasive or in situ breast carcinoma frequently report that concerns regarding disease recurrence and the side effects of radiation influenced their treatment decisions, and that these concerns led them to favor mastectomy.15 Important prognostic factors in local recurrence for patients with DCIS treated with BCS include age, population, tumor size, histologic grade, comedonecrosis, margin width and the Van Nuys Prognostic Index (VNPI).16-19 Kim, et al.20 showed that younger age at diagnosis and the omission of adjuvant RT independently predict recurrence in Korean patients with DCIS who are treated with BCS.
On the other hands, Punglia, et al.21 have recently showed that patient age and preference should be considered when making the decision to add or not to add radiation for DCIS.
Among these factors, Schwartz, et al.22,23 and Fisher, et al.17 emphasized the significance of comedonecrosis in predicting local recurrence. Schwartz, et al. determined that patients with small non-comedo lesions with uninvolved margins are not likely to receive any significant benefits from RT, and that patients with DCIS having calcifications ranging from 2.0 to 2.5 cm in diameter may, safely choose to omit RT with continual surveillance. Based on the results from the National Surgical Adjuvant Breast and Bowel Project (NSABP) protocol B-24, which included 1456 patients with 10.5 years of follow-up, Fisher, et al. concluded that ductal comedonecrosis, micropapillary histologic tumor type, and multifocality predict IBTR strongly and independently, and that data from the NSABP B-17, which included 2079 patients, identified comedonecrosis as a simple strong predictor for IBTR.
Gilleard, et al.18 analyzed 5 randomized controlled clinical trials and 64 observational studies published from 1970 to 2009 to test the associations of patient and tumor characteristics with clinical outcomes in women with DCIS, and reported that tumor size was positively associated with IBTR; however, many of the relationships tested in these studies were not statistically significant. The studies generally classified tumors of less than 20 mm as small, although some defined small as less than 5 mm.
Surgical margin is an another important risk factor for recurrence. In early 1990, Veronesi, et al.24 reported that tumor excision with 2-3 cm of normal tissue around the infiltrating tumor resulted in a lower local recurrence rate than excision with 1 cm of normal tissue. Silverstein, et al.11 suggested that for patients with DCIS treated with BCS, excellent control of local recurrence can be achieved without radiation therapy if the tumor is excised with a margin of at least 10 mm, regardless of nuclear grade, the presence of comedonecrosis, or tumor size.
When we initially designed this study, no specific criteria for omission of RT, analogous to the Van Nuys DCIS Pathologic Classification or the VNPI,16 had yet been proposed. Therefore, we postulated that the absence of comedonecrosis, a tumor size less than 2 cm, and a sufficiently large excision margin would provide valid criteria for the omission of adjuvant RT after PM in DCIS.
The results of several prospective trials show that adjuvant RT after BCS significantly reduces the risk of local recurrence in DCIS. Nevertheless, in contrast to data for patients with invasive breast cancer, who show a significant survival benefit from RT after BCS,25,26 none of these randomized trials show a benefit for patients with DCIS in terms of distant metastases, breast cancer-specific survival, or overall survival.4-9 Moreover, although the risk of complications after adjuvant RT is small,27 such risk must be considered to determine the subsets of patients with DCIS most likely to benefit from RT.28 Gilleard, et al.29 emphasized the following four risks after RT: 1) increased mortality from lung cancer and cardiac disease, 2) loss of option to use RT for IBTR, 3) increased difficulty in mammographic follow-up, which might delay diagnosis, and 4) a greater probability of invasiveness in a tumor that recurs after RT.
Unfortunately, within these large trials, no subset analyses were performed to identify patients for whom adjuvant RT might be safely omitted. In a prospective study, Hughes, et al.30 did successfully identify such a group of low-risk patients with DCIS. The authors reported a 5-year rate of ipsilateral breast events (IBEs) of 6.1% in 565 eligible patients with tumors 2.5 cm or smaller and a low- to intermediate-grade stratum, and 15.3% recurrence in 105 eligible patients with tumors 1.0 cm or smaller and a high-grade stratum. All patients had a microscopic margin of 3 mm or greater. The authors concluded that patients rigorously evaluated and selected for low- to intermediate-grade DCIS with margins 3 mm or wider had an acceptably low rate of ipsilateral breast events at 5 years after excision to omit the post-operative irradiation. The higher recurrence rate among patients with high-grade lesions suggested that excision alone does not adequately treat these lesions.
Although clinically significant as a first prospective trial of outcome following omission of RT, the study by Hughes, et al. attracted sharp criticism. Harris and Morrow31 disputed the results, first because the IBEs began to increase after 5 years, especially among patients with low- to intermediate-grade DCIS, and also because patients involved in this trial had overall a more favorable lesion size and margin width than specified by the inclusion criteria. Motwani, et al.32 showed that adjuvant whole breast RT, with a boost to the median total tumor bed dose of 64 Gy, reduced the rate of local recurrence by more than 70% (from 6.1% to 1.5% in the low/intermediate cohort, and from 15.3% to 2% in the high-grade cohort for the 5-year IBTR rate) and produced no differences in the incidence of contralateral breast cancer among patients with DCIS who met the Eastern Cooperative Oncology Group Study 5194 (E5194) criteria.
In the relatively long follow-up period of the present study (mean 84 months), we observed no case of local recurrence, distant metastasis, or mortality among DCIS patients who met our criteria. Interestingly, 7 patients (8 breasts) for whom adjuvant RT was omitted in spite of the presence of comedonecrosis, had no IBTR; all of these patients had relatively small lesions (mean 0.95 cm, range 0.67-1.5 cm). Within the limitations of this study, this outcome may tentatively be interpreted to mean that small lesion size (less than 1.5 cm) and/or the absence of comedonecrosis, combined with a relatively large resection margin, constitute acceptable criteria for omission of adjuvant RT following PM for DCIS. This set of criteria may have an advantage over the VNPI for its relative simplicity, if validated in further trials.
This study is limited first and most importantly, by the small number of subjects and events included in the analysis, and the use of a retrospective design rather than a prospective randomized design, which would greatly reduce selection bias. As Hughes, et al. point out, a more favorable tumor size among study subjects than specified by inclusion criteria (mean 0.60 cm without comedonecrosis, and 0.95 cm with comedonecrosis in our study) may introduce a positive bias for outcome in the subset of patients for whom RT was omitted. Although events affecting outcome may increase after 7 years, we would need to follow a larger patient sample for 10 years or more to determine this. In addition, a margin of 2 cm or more may be somewhat excessive to maintain an aesthetic contour, which for some patients may be as important to consider as the oncologic outcome.
In conclusion, based on this study, we propose a relatively simple set of criteria to determine whether adjuvant RT may be safely omitted after PM for some Korean patients with DCIS. These criteria include the absence of comedonecrosis, lesion size 2 cm or less, and an excision margin greater than 2 cm. Even in the presence of comedonecrosis, omission of adjuvant RT may be considered for patients with lesions of 1.5 cm or less, if a wide excision margin was achieved. To verify the safety of these criteria and the acceptability of outcome, a large prospective randomized trial is needed. Future investigations must also address the molecular aspects of this approach to treat DCIS, so as to predict outcome more precisely and optimize the treatment for each individual.
Figures and Tables
 | Fig. 1Disease-free survival for local recurrence in patients with ductal carcinoma in situ treated with partial mastectomy without radiotherapy. |
 | Fig. 2Disease-free survival for contralateral breast events according to comedo-necrosis in patients with ductal carcinoma in situ treated with partial mastectomy without radiotherapy. |
Table 1
Clinicopathologic Characteristics of 26 Breasts (25 Patients) Treated with Partial Mastectomy without Adjuvant Radiotherapy for Ductal Carcinoma In Situ
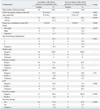
HER2, human epidermal growth factor receptor 2.
*HER2 and p53 were tested by immunohistochemical staining (IHC). HER2-positive status was confirmed if the IHC staining showed a positive result in three tests or if fluorescence in situ hybridization showed positive gene amplification for three non-positive IHC results.
References
1. Ko SS. Korean Breast Cancer Society. Chronological changing patterns of clinical characteristics of Korean breast cancer patients during 10 years (1996-2006) using nationwide breast cancer registration on-line program: biannual update. J Surg Oncol. 2008. 98:318–323.

2. Kerlikowske K. Epidemiology of ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010. 2010:139–141.

3. Silverstein MJ, Barth A, Poller DN, Gierson ED, Colburn WJ, Waisman JR, et al. Ten-year results comparing mastectomy to excision and radiation therapy for ductal carcinoma in situ of the breast. Eur J Cancer. 1995. 31A:1425–1427.

4. Fisher ER, Dignam J, Tan-Chiu E, Costantino J, Fisher B, Paik S, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17:intraductal carcinoma. Cancer. 1999. 86:429–438.

5. Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001. 28:400–418.

6. Wapnir I, Dignam J, Julian TB, Land S, Mamounas EP, Anderson S, et al. Long-term outcomes after invasive breast tumor recurrence (IBTR) in women with DCIS in NSABP B-17 and B-24. J CLIN ONCOL (MEETING ABSTRACTS). 2007. 25:520. abstr 520.

7. EORTC Breast Cancer Cooperative Group. EORTC Radiotherapy Group. Bijker N, Meijnen P, Peterse JL, Bogaerts J, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853--a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006. 24:3381–3387.

8. Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003. 362:95–102.

9. Ringberg A, Nordgren H, Thorstensson S, Idvall I, Garmo H, Granstrand B, et al. Histopathological risk factors for ipsilateral breast events after breast conserving treatment for ductal carcinoma in situ of the breast--results from the Swedish randomised trial. Eur J Cancer. 2007. 43:291–298.

10. Morrow M, Strom EA, Bassett LW, Dershaw DD, Fowble B, Harris JR, et al. Standard for the management of ductal carcinoma in situ of the breast (DCIS). CA Cancer J Clin. 2002. 52:256–276.

11. Silverstein MJ, Lagios MD, Groshen S, Waisman JR, Lewinsky BS, Martino S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999. 340:1455–1461.

12. Macdonald HR, Silverstein MJ, Lee LA, Ye W, Sanghavi P, Holmes DR, et al. Margin width as the sole determinant of local recurrence after breast conservation in patients with ductal carcinoma in situ of the breast. Am J Surg. 2006. 192:420–422.

13. Viani GA, Stefano EJ, Afonso SL, De Fendi LI, Soares FV, Leon PG, et al. Breast-conserving surgery with or without radiotherapy in women with ductal carcinoma in situ: a meta-analysis of randomized trials. Radiat Oncol. 2007. 2:28.

14. Rourke LL, Hunt KK. Avoiding radiation after breast-conserving surgery for ductal carcinoma in situ of the breast: beyond the margin. Ann Surg. 2010. 251:592–594.

15. Katz SJ, Lantz PM, Janz NK, Fagerlin A, Schwartz K, Liu L, et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol. 2005. 23:5526–5533.

16. Silverstein MJ, Lagios MD, Craig PH, Waisman JR, Lewinsky BS, Colburn WJ, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer. 1996. 77:2267–2274.

17. Fisher ER, Land SR, Saad RS, Fisher B, Wickerham DL, Wang M, et al. Pathologic variables predictive of breast events in patients with ductal carcinoma in situ. Am J Clin Pathol. 2007. 128:86–91.

18. Gilleard O, Goodman A, Cooper M, Davies M, Dunn J. The significance of the Van Nuys prognostic index in the management of ductal carcinoma in situ. World J Surg Oncol. 2008. 6:61.

19. Shamliyan T, Wang SY, Virnig BA, Tuttle TM, Kane RL. Association between patient and tumor characteristics with clinical outcomes in women with ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010. 2010:121–129.

20. Kim JS, Moon HG, Ahn SK, Min JW, Shin HC, Kim HS, et al. Clinicopathological characteristics and factors affecting recurrence of ductal carcinoma in situ in Korean women. J Breast Cancer. 2010. 13:392–397.

21. Punglia RS, Burstein HJ, Weeks JC. Radiation therapy for ductal carcinoma in situ: a decision analysis. Cancer. 2012. 118:603–611.
22. Schwartz GF, Finkel GC, Garcia JC, Patchefsky AS. Subclinical ductal carcinoma in situ of the breast. Treatment by local excision and surveillance alone. Cancer. 1992. 70:2468–2474.

23. Schwartz GF. The role of excision and surveillance alone in subclinical DCIS of the breast. Oncology (Williston Park). 1994. 8:21–26.
24. Veronesi U, Volterrani F, Luini A, Saccozzi R, Del Vecchio M, Zucali R, et al. Quadrantectomy versus lumpectomy for small size breast cancer. Eur J Cancer. 1990. 26:671–673.

25. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005. 366:2087–2106.

26. Lee HD, Yoon DS, Koo JY, Suh CO, Jung WH, Oh KK. Breast conserving therapy in stage I & II breast cancer in Korea. Breast Cancer Res Treat. 1997. 44:193–199.

27. Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001. 344:1997–2008.

28. Lagios MD, Silverstein MJ. Ductal carcinoma in situ: dilemma or denouement. J Clin Oncol. 2010. 28:e218–e219.

29. Gilleard O, Davies M, Dunn J. Is it safe to omit radiotherapy following wide local excision for ductal carcinoma in situ? Surgeon. 2009. 7:146–150.

30. Hughes LL, Wang M, Page DL, Gray R, Solin LJ, Davidson NE, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009. 27:5319–5324.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download