Abstract
Purpose
There is a lack of sufficient data in comparison of optical coherence tomographic (OCT) findings between first- and second-generation drug-eluting stents (DES). Compared to first-generation (i.e., sirolimus- or paclitaxel-eluting stents), second-generation DESs (i.e., everolimus- or biolinx-based zotarolimus-eluting stents) might have more favorable neointimal coverage.
Materials and Methods
Follow-up OCT findings of 103 patients (119 lesions) treated with second-generation DESs were compared with those of 139 patients (149 lesions) treated with first-generation DESs. The percentage of uncovered or malapposed struts, calculated as the ratio of uncovered or malapposed struts to total struts in all OCT cross-sections, respectively, was compared between the two groups.
Results
Both DES groups showed similar suppression of neointimal hyperplasia (NIH) on OCT (mean NIH cross-sectional area; second- vs. first-generation=1.1±0.5 versus 1.2±1.0 mm2, respectively, p=0.547). However, the percentage of uncovered struts of second-generation DESs was significantly smaller than that of first-generation DESs (3.8±4.8% vs.7.5±11.1%, respectively, p<0.001). The percentage of malapposed struts was also significantly smaller in second-generation DESs than in first-generation DESs (0.4±1.6% vs.1.4±3.7%, respectively, p=0.005). In addition, intra-stent thrombi were less frequently detected in second-generations DESs than in first-generation DESs (8% vs. 20%, respectively, p=0.004).
With the introduction of first-generation drug-eluting stents (DES), a significant reduction in restenosis rates and an improvement in short-term clinical outcomes were reported.1,2 However, the use of first-generation DESs, e.g., sirolimus- and paclitaxel-eluting stents, has been shown to be strongly related with the occurrence of late or very late stent thrombosis, raising safety concerns.3,4 Several attempts to develop newer DESs have set out to prevent the occurrence of stent thrombosis by modifying the eluted drugs, drug carrying systems, and stent design. Of these, second-generation DESs, e.g., everolimus-eluting stents (EES) and Biolinx-based zotarolimus-eluting stents (Bx-ZES), have been reported to suppress neointima hyperplasia (NIH) effectively and simultaneously demonstrate favorable long-term outcomes.5-9 Optical coherence tomography (OCT) has enabled researchers to evaluate neointimal coverage of DESs in detail, even at the strut level.10,11 However, no sufficient OCT data has been reported comparing neointimal coverage between first- and second-generation DESs. Therefore, using OCT, we sought to compare healing responses, including neointimal coverage, between the two groups.
We used data submitted to the Yonsei OCT registry, evaluating neointimal coverage in patients who underwent coronary stent implantation for de novo lesions.12,13 General exclusion criteria for the follow-up OCT study were as follows: 1) untreated significant left main coronary artery disease, 2) apparent congestive heart failure, 3) renal insufficiency (baseline creatinine ≥2.0 mg/dL), and 4) lesions unsuitable for OCT imaging (vessel size ≥3.5 mm or lesions within 10 mm of the ostium of a major epicardial artery). Between September 2007 and October 2010, a total of 242 patients with 268 lesions were selected from the OCT registry database. Inclusion criteria of the current study comprised lesions treated with EES, Bx-ZES, sirolimus- or paclitaxel-eluting stents, as well as those followed with a follow-up OCT examination at 12±4 months after stent implantation. Exclusion criteria were 1) bifurcation treated with 2-stent techniques, 2) angiographic evidence of restenosis, 3) lesions with repeated revascularization, 4) bare-metal stent implantation, and 5) poor OCT image quality. Second-generation DESs were deployed in 103 patients for 119 lesions including EES (Xience V™, Abbott Vascular, Santa Clara, CA, USA) and Bx-ZES (Endeavor Resolute®, Medtronic, Santa Rosa, CA, USA), while first-generation DESs were implanted in 139 patients for 149 lesions including sirolimus-eluting stents (Cypher™, Cordis, Miami, FL, USA) and paclitaxel-eluting stents (Taxus™, Boston scientific, Natick, MA, USA). DES implantation was performed using current, conventional techniques, and the choice of DES was made according to the operators' discretion. After DES implantation, all patients received dual antiplatelet therapy with aspirin and clopidogrel until the follow-up OCT was conducted. This study was approved by the Institutional Review Board of our institute, and written informed consent was obtained from each patient.
Detailed explanations regarding the OCT system and methods for imaging have been described in our previous studies.12,13 OCT examination was performed using a conventional OCT system (Model M2 Cardiology Imaging System, LightLab Imaging, Westford, MA, USA) with a motorized pull-back system at 1.0 mm/s. The occlusion catheter was positioned proximal to the stent, and a 0.014-inch wire-type imaging catheter (ImageWire, LightLab Imaging) was positioned distal to the stent. During image acquisition, the occlusion balloon (Helios, Avantec Vascular, Sunnyvale, CA, USA) was inflated to 0.4-0.6 atm, and lactated Ringer's solution was infused at a rate of 1.0 mL/s. The imaging wire was pulled from distal to proximal, and continuous images were acquired and stored digitally for subsequent analysis.12,13 OCT analysis was performed by an independent investigator blinded to patient and procedural information.
Cross-sectional OCT images were analyzed at 1-mm intervals (every 15 frames). Stent and luminal cross-sectional areas (CSAs) were measured at 1-mm intervals, and NIH CSA was calculated as the stent CSA minus the luminal CSA. Percent NIH CSA was calculated as NIH CSA×100/stent CSA. Mean values are reported in this study. The thickness of NIH, defined as the distance between the endoluminal surface of neointima and the strut, was measured inside the struts at a line as perpendicular as possible to the neointima and strut.10 An uncovered strut was defined as having a NIH thickness of 0 µm.10,14 A malapposed strut was defined as a strut that had detached from the vessel wall (Cypher™, ≥160 µm; Taxus™, ≥130 µm; Endeavor Resolute®, ≥110 µm; Xience™, ≥100 µm).15,16 The percentage of uncovered or malapposed struts was investigated for evaluation of the healing responses of DESs as shown on OCT. The percentage of malapposed or uncovered struts in each stented lesion was calculated as the (number of malapposed or uncovered struts/total number of struts in all cross-sections of the lesion)×100, respectively. Cross sections with major side branches (diameter ≥2 mm) were excluded from this analysis. The neointimal coverage of stent struts in each cross-section was evaluated, and then the percentage of uncovered struts was analyzed and compared between the two groups. Intra-stent thrombi were defined as a signal-rich, low-backscattering protrusions or high-backscattering protrusions inside the lumen of the artery with signal-free shadowing on OCT images (dimension ≥250 µm).13
Quantitative coronary angiography analysis was performed using an offline quantitative coronary angiography system (CASS system II, Pie Medical Imaging, Nuenen, the Netherlands) before and after stent implantation and at the follow-up angiogram. The minimal luminal diameter of the coronary lesions treated with DESs and a reference diameter were measured in the view that was the most severe and not foreshortened.
All statistical analyses were performed using the Statistical Analysis System software (SAS; 9.1.3., SAS Institute, Cary, NC, USA). Categorical data were presented as a number (%) and compared with Chi-square statistics or Fisher's exact test. Continuous data were presented as mean±standard deviation and compared with Student's t-test. Comparisons among the four different DESs were performed by analysis of variance with post-hoc analysis by the Bonferroni method. If the distributions were skewed, a non-parametric test was used. A value of p<0.05 was considered statistically significant.
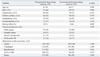
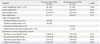
Baseline clinical characteristics are shown in Table 1. There were no significant differences in baseline clinical characteristics between the two groups. Baseline angiographic and procedural characteristics are listed in Table 2. There were also no significant differences in baseline angiographic and procedural characteristics between the two groups.
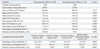
Table 3 summarizes the OCT findings of both groups. There were no significant differences in mean lumen and stent CSA, as well as time to follow-up OCT (days) between the two groups. Although both groups showed a nearly similar suppression of NIH, the percentage of uncovered struts of second-generation DESs was significantly smaller than that of first-generation DESs (3.8±4.8% vs. 7.5±11.1%, respectively, p<0.001). The percentage of malapposed struts was also significantly smaller in second-generation DESs than in first-generation DESs (0.4±1.6% vs. 1.4±3.7%, respectively, p=0.005). In addition, intra-stent thrombi were less frequently detected in second-generations DESs than in first-generation DESs (8% vs. 20%, respectively, p=0.004).
This follow-up OCT study demonstrated that second-generation DESs lead to a lower percentage of uncovered and malapposed struts, as well as a lower incidence of intra-stent thrombi, compared with first-generation DESs. In spite of superior healing responses of second-generation DESs, the efficacy of second-generation DESs in the suppression of NIH growth was not different from that of first-generation DESs.
As concerns regarding the safety issues of first-generation DESs have increased, newly developing DESs have focused more on safety with a similar efficacy to that of first-generation DESs through the long-term follow-up studies.3,4 Among many newly developed DESs, EES and Bx-ZES have been shown to have both excellent efficacy and safety.5-9 Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) III randomized study showed that EES implantation resulted in a statistically significant reduction of angiographic late loss at 8-month follow-up and showed significantly improved event-free survival, compared with implantation of paclitaxel-eluting stents at 2-year follow-up.5,6 In addition, compared with patients treated with paclitaxel-eluting stents, those treated with EES tended to have fewer episodes of late stent thrombosis at 1 and 2 years (0.2% versus 1.0%; p=0.10) thereafter.6 Recently, Bx-ZES, which comprises a low-profile, thin-strut platform, and a Biolinx tri-polymer, also showed favorable short-term angiographic outcomes with a comparable low late loss.7
Many potential mechanisms or factors are expected to be related with the favorable outcomes demonstrated in second-generation DESs.5-8,17 Of these, the degree of reendothelialization, regarded as the most powerful predictor of stent thrombosis, might be strongly related with better outcomes in DESs.11,18,19 Although some studies have evaluated neointimal coverage for various types of DES, they were conducted as an autopsy or animal study.17,18 A new imaging tool, OCT, has enabled researchers to evaluate the reendothelialization of DES in live patients with a superior resolution capacity.10,11 Therefore, using OCT, healing responses, including neointimal coverage of stent struts, was evaluated between second-generation and first-generation DESs in this study.
In the current OCT study, the rate of uncovered struts as shown on OCT was significantly different between the two groups; second-generation DESs showed a higher % of uncovered struts, meaning more complete neointimal coverage. However, the amount of NIH was similar between the first- and second-generation DESs. The superior nature of second-generation DESs, showing better endothelialization and healing responses compared with first-generation DESs, while maintaining similar efficacy represented by the suppression of NIH on OCT or a low late loss on follow-up angiogram, might be caused by the unique components comprising DESs, including the use of novel drugs, superior biocompatibility and morphology of polymers, reduced polymer layers, and thin-strut design.17 As a result, newer DESs are both safe and have equal efficacy to first-generation DESs, and this study, in comparison of the OCT findings thereof, suggests that second-generation DESs are close to the ideal DES.
The degree of stent malapposition, evaluated by OCT, was also significantly different between the two groups; the percentage of malapposed struts of second-generation DESs was significantly lower than that of first-generation DESs. Stent malapposition has been also regarded as an important predictor of DES thrombosis in intravascular ultrasound studies.20,21 DES type has been suggested as one of the most determining factors of stent malapposition.21,22 A difference in the incidence of stent malapposition dependent upon the type of DES might be associated with differences in long-term outcomes. Namely, a lower rate of malapposition in second-generation DESs may translate to more favorable clinical outcomes after DES implantation thereof.
This study has several limitations. First, selection bias might affect the results because this was a non-randomized registry study. Second, because this study was not a controlled comparative one, direct comparisons among four different DESs could not be performed. However, there were no significant differences in the baseline clinical and angiographic parameters between the two groups. Third, because the study population of the current study was free of major adverse events after DES implantation until follow-up OCT, the lesions of this study might not represent those seen in real world practice. Fourth, in this study, the first-generation DES group, which consisted of sirolimus-eluting stents and paclitaxel-eluting stents, was compared with second-generation DESs. However, because sirolimus-eluting stents and paclitaxel-eluting stents showed different outcomes in some pathologic and imaging studies,12,13,23,24 careful attention must be given when interpreting the results. Finally, no clinical follow-up data was provided due to the short duration of clinical follow-up after OCT evaluation.
In conclusion, this follow-up OCT study showed that second-generation DESs characteristically had greater neointimal coverage than first-generation DESs. For more definite conclusions, long term clinical and serial OCT follow-up with a larger population will be needed in the future.
Figures and Tables
ACKNOWLEDGEMENTS
This study was partly supported by grants from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (No. A085012 and A102064), the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (No. A085136), and the Cardiovascular Research Center, in Seoul, Korea.
References
1. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003. 349:1315–1323.

2. Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004. 350:221–231.

3. Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007. 356:998–1008.

4. Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007. 369:667–678.

5. Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008. 299:1903–1913.

6. Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, et al. Randomized comparison of everolimus-eluting and paclitaxel-eluting stents: two-year clinical follow-up from the Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) III trial. Circulation. 2009. 119:680–686.

7. Meredith IT, Worthley S, Whitbourn R, Walters DL, McClean D, Horrigan M, et al. Clinical and angiographic results with the next-generation resolute stent system: a prospective, multicenter, first-in-human trial. JACC Cardiovasc Interv. 2009. 2:977–985.

8. Mukherjee D, Moliterno DJ. Second-generation drug-eluting stents and the continuous need for rapidly available real-world data. JACC Cardiovasc Interv. 2009. 2:1236–1239.

9. Meredith IT, Worthley SG, Whitbourn R, Walters D, McClean D, Ormiston J, et al. Long-term clinical outcomes with the next-generation Resolute Stent System: a report of the two-year follow-up from the RESOLUTE clinical trial. EuroIntervention. 2010. 5:692–697.

10. Takano M, Inami S, Jang IK, Yamamoto M, Murakami D, Seimiya K, et al. Evaluation by optical coherence tomography of neointimal coverage of sirolimus-eluting stent three months after implantation. Am J Cardiol. 2007. 99:1033–1038.

11. Guagliumi G, Sirbu V. Optical coherence tomography: high resolution intravascular imaging to evaluate vascular healing after coronary stenting. Catheter Cardiovasc Interv. 2008. 72:237–247.

12. Kim U, Kim JS, Kim JS, Lee JM, Son JW, Kim J, et al. The initial extent of malapposition in ST-elevation myocardial infarction treated with drug-eluting stent: the usefulness of optical coherence tomography. Yonsei Med J. 2010. 51:332–338.

13. Kim JS, Hong MK, Fan C, Kim TH, Shim JM, Park SM, et al. Intracoronary thrombus formation after drug-eluting stents implantation: optical coherence tomographic study. Am Heart J. 2010. 159:278–283.

14. Barlis P, Dimopoulos K, Tanigawa J, Dzielicka E, Ferrante G, Del Furia F, et al. Quantitative analysis of intracoronary optical coherence tomography measurements of stent strut apposition and tissue coverage. Int J Cardiol. 2010. 141:151–156.

15. Tanigawa J, Barlis P, Di Mario C. Intravascular optical coherence tomography: optimisation of image acquisition and quantitative assessment of stent strut apposition. EuroIntervention. 2007. 3:128–136.
16. Tanigawa J, Barlis P, Dimopoulos K, Dalby M, Moore P, Di Mario C. The influence of strut thickness and cell design on immediate apposition of drug-eluting stents assessed by optical coherence tomography. Int J Cardiol. 2009. 134:180–188.

17. Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008. 52:333–342.

18. Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007. 115:2435–2441.

19. Cook S, Wenaweser P, Togni M, Billinger M, Morger C, Seiler C, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation. 2007. 115:2426–2434.

20. Hassan AK, Bergheanu SC, Stijnen T, van der Hoeven BL, Snoep JD, Plevier JW, et al. Late stent malapposition risk is higher after drug-eluting stent compared with bare-metal stent implantation and associates with late stent thrombosis. Eur Heart J. 2010. 31:1172–1180.

21. Hong MK, Mintz GS, Lee CW, Park DW, Park KM, Lee BK, et al. Late stent malapposition after drug-eluting stent implantation: an intravascular ultrasound analysis with long-term follow-up. Circulation. 2006. 113:414–419.

22. Kim JS, Jang IK, Kim JS, Kim TH, Takano M, Kume T, et al. Optical coherence tomography evaluation of zotarolimus-eluting stents at 9-month follow-up: comparison with sirolimus-eluting stents. Heart. 2009. 95:1907–1912.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download