Abstract
Purpose
We investigated correlations of coronary plaque composition determined by virtual histology (VH) intravascular ultrasound (IVUS) and blood levels of biomarkers that represent the vulnerability of coronary plaques.
Materials and Methods
Pre- and postprocedural blood levels of high sensitivity C-reactive protein, soluble CD40 ligand (sCD40L), matrix metalloproteinase-9, and neopterin were measured in 70 patients with stable angina (SA) or unstable angina (UA) who were undergoing percutaneous coronary intervention (PCI) for single lesions. We evaluated the data for correlations between these biomarkers and necrotic core contents in PCI target lesions analyzed by VH.
Results
Clinical characteristics, IVUS, VH, and biomarker blood levels were not different between the SA and the UA group except for more frequent previous statin use (52.3% vs. 23.1%, p=0.017) and lower remodeling index in the SA group (0.98±0.09 vs. 1.10±0.070, p<0.001). Among the biomarkers evaluated, only pre-PCI neopterin level showed a weakly significant correlation with the absolute volume of the necrotic core (r=0.320, p=0.008). Pre- and post-PCI blood levels of sCD40L (r=0.220, p=0.072; r=0.231, p=0.062) and post-PCI blood level of neopterin (r=0.238, p=0.051) showed trends toward weakly positive correlations with the absolute volume of necrotic core.
The process of coronary plaque destabilization, rupture, and thrombosis is associated with the development of acute coronary syndrome (ACS).1 Vulnerable atherosclerotic plaques are prone to rupture and are generally characterized by a large lipid core, the presence of inflammatory cells, a paucity of smooth muscle cells and fibrous tissue, thinning of the fibrous cap, positive remodeling, and intimal neovascularization.1-3 Various molecules of inflammation and tissue proteolysis are activated in the formation of vulnerable plaques.4-6 C-reactive protein (CRP), soluble CD40 ligand (sCD40L), matrix metalloproteinase (MMP), and neopterin are known to be involved in the process, and their blood levels are elevated in patients with ACS.7-11
Virtual histology (VH) of intravascular ultrasounds (IVUS) (Volcano Therapeutics, Inc., Rancho Cordova, CA, USA) was developed to assess the tissue compositions of coronary plaques. VH uses spectral analysis of radiofrequency ultrasound signals and classifies plaques into four major components: fibrous, fibro-fatty, necrotic core, and dense calcium.12,13 Previous studies found that culprit lesions in patients with ACS have greater amounts of necrotic core than with target lesions in patients with stable angina (SA), and that necrotic core volume predicts microembolism and no reflow during percutaneous coronary intervention (PCI).14-16
Therefore, the purpose of the present study was to investigate whether the relative content of necrotic core, determined by VH, is correlated with periprocedural blood levels of various biomarkers that represent the vulnerability of coronary plaques in patients undergoing PCI.
A total of 70 consecutive patients with SA or unstable angina (UA), participating in the Real Safety and Efficacy of a 3-month Dual Antiplatelet Therapy Following Zotarolimus-eluting Stents Implantation (RESET) trial who underwent PCI at Severance Cardiovascular Hospital, Yonsei University Health System, Seoul, Korea, were enrolled in this study. Inclusion criteria were age >20 years, the presence of a single significant coronary artery stenosis (>50%) with a clinical presentation of stable or unstable angina, and the presence of a PCI target lesion with a reference diameter of 2.5-4 mm and a lesion length ≤26 mm that could be covered by a single stent. Exclusion criteria were hemodynamic instability, heart failure (left ventricular ejection fraction <40%), history of stroke, peripheral artery disease, significant hepatic (aspartate aminotransferase or alanine aminotransferase ≥3 times the upper limit of normal range) or renal failure (serum creatinine >2.0 mg/dL), previous use of drug-eluting stents, left main disease, bifurcation lesion requiring 2-stent technique, chronic total occlusion, restenotic lesion, all women with childbearing potential or women with pregnancy, known bleeding diathesis or bleeding history within the previous 3 months, and contraindications to antiplatelet agents. We also excluded patients with acute myocardial infarction because in these patients the blood levels of biomarkers might be influenced not only by the vulnerability of coronary plaques but also by the extent of myocardial necrosis. In addition, we excluded patients who took corticosteroids or other anti-inflammatory drugs except low-dose aspirin, and patients with any identifiable condition that was related to infectious, active allergic, connective tissue-related, or other chronic inflammatory diseases febrile conditions, acute infection, or known chronic inflammatory disease. Informed written consent was obtained from all patients, and the study protocol was approved by the institutional review board of Yonsei University Health System in accordance with the Declaration of Helsinki.
SA was defined as no change in the frequency, duration, or intensity of symptoms within 6 weeks before the intervention. UA was defined as chest pain occurring at rest and lasting longer than 20 min, new-onset chest pain of at least Canadian Cardiovascular Society class III severity, or previously diagnosed angina that has become distinctly more frequent, longer in duration, or lower in threshold.
All patients were placed on chronic aspirin (100-325 mg/day) and clopidogrel (75 mg/day) therapy for ≥5 days or received aspirin (250 mg) and clopidogrel (300-600 mg) loading at least 12 hours before PCI. Unfractionated heparin was administered according to the local standards of care (target activated clotting time >250 sec) at a dosage consonant with label instructions. In all patients, PCI target lesions were predilated prior to stenting and treated with drug-eluting stents such as a zotarolimus-eluting stent (Endeavor Sprint® or Endeavor Resolute®, Medtronic Vascular Inc., Santa Rosa, CA, USA), sirolimus-eluting stent (Cypher®, Cordis, Johnson & Johnson, Miami Lakes, FL, USA) or everolimus-eluting stent (Xience V®, Abbott Vascular, Santa Clara, CA, USA). After implantation of the stent, aspirin (100 mg/day) was administered indefinitely, and clopidogrel (75 mg/day) was given for 3 or 12 months according to the RESET study protocol.
IVUS investigations were performed in all PCI target lesions prior to predilation using a 20-MHz 2.9F, phased-array IVUS catheter (Eagle Eye, Volcano Therapeutics, Rancho Cordova, CA, USA). After intracoronary administration of nitroglycerin (200 µg), the IVUS catheter was placed distal to the target lesion and then pulled back using a motorized pullback system at 0.5 cm/s. During pullback, gray-scale IVUS was recorded and raw radiofrequency data were captured at the top of the R wave for reconstruction of the color-coded map by a VH-IVUS data recorder (Volcano Therapeutics). Gray-scale quantitative IVUS analyses of external elastic membrane, lumen, plaque, and media (P&M, external elastic membrane-lumen) were performed according to the Clinical Expert Consensus Document on IVUS.17 The remodeling index for the PCI target lesion was defined as the ratio of the external elastic membrane (EEM) area at the cross-section with minimum luminal area to the reference EEM area (average of the proximal and distal reference segments). VH data were analyzed for the site of the minimal lumen area and the whole length of the target lesion at 1-mm intervals using pcVH software (Volcano Therapeutics). VH analysis coded tissue as green (fibrotic), yellow-green (fibro-fatty), white (dense calcium), or red (necrotic core).12,13 VH analyses were reported in absolute amounts and as percentages (relative amounts) of plaque area or volume.
Blood samples were obtained before bolus administration of heparin and PCI in the catheterization room and 24 h after PCI. The blood samples were centrifuged immediately, and both plasma and serum were aliquoted and stored at -80℃ for subsequent analysis. Investigational biomarkers, including high sensitivity CRP (hsCRP), MMP-9, sCD40L, and neopterin, were measured at an external clinical laboratory (Seoul Clinical Laboratories, Seoul, Korea). Serum hsCRP was measured using a latex-enhanced turbidimetric immunoassay. The serum levels of MMP-9 and plasma levels of sCD40L and neopterin were determined using Quantikine® immunoassays (R&D Systems, Minneapolis, MN, USA), which use a quantitative sandwich enzyme immunoassay technique.
All analyses were performed using SPSS version 18.0 statistical software (SPSS Inc., Chicago, IL, USA). Categorical variables were expressed as percentages and compared using chi-square tests. When the expected cell number was <5, Fisher's exact tests were used. Continuous data were expressed as mean±SD. Continuous variables were analyzed using unpaired t-tests and the Mann-Whitney U-test, as appropriate. Categorical data and proportions were analyzed using the χ2-test or Fisher's exact test, as required. Pearson's two-way test was used to assess the relationships between two quantitative variables with normal distributions. For all tests, two-tailed p values <0.05 were considered significant.
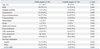
Among the 70 enrolled patients (age 60.4±8.9 years, male 57.1%), there were 26 UA and 44 SA patients. There were no significant differences between the two patient groups in baseline characteristics such as age, gender, diabetes mellitus, hypertension, hypercholesterolemia, current smoking, renal failure, previous stroke, and multivessel disease (Table 1). However, the SA group had a higher frequency of previous statin use (52.3% vs. 23.1%, p=0.017). Consequently, total cholesterol (170.4±37.7 vs. 194.6±38.2 mg/dL, p=0.013) and low density lipoprotein cholesterol levels (95.5±34.1 vs. 114.9±37.4 mg/dL, p=0.032) were lower in the SA group than in the UA group.
Angiographic characteristics and procedural data did not differ significantly between the UA and SA patient groups and are summarized in Table 2. The most common target lesion location was the left anterior descending artery. The majority of the lesions were type B2 or C lesions according to the American College of Cardiology/American Heart Association classification. All lesions were successfully treated with a single drug-eluting stent.
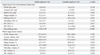
Preprocedural IVUS and VH data are presented in Table 3. There were no significant differences in vessel, lumen, and P&M area at the target lesion cross section with the minimum lumen area or in the vessel, lumen, and P&M volume of the target lesion between the SA and UA patient groups. VH also demonstrated no significant intergroup differences in plaque composition at the target lesion cross section with minimum lumen area or in the tissue component of the whole target lesion. However, patients with UA showed a trend toward greater necrotic volume (9.4±3.8% vs. 7.5±3.7%, p=0.052) and less fibro-fatty tissue (15.1±8.1% vs. 18.3±7.6%, p=0.110) than SA patients. The remodeling index was significantly greater for the UA group than the SA group (1.10±0.070 vs. 0.98±0.09, p<0.001).
Pre- and postprocedural blood levels of hsCRP, sCD40L, MMP-9, and neopterin are shown in Table 4. Between the SA and UA patient groups, there were no significant differences in pre- and postprocedural blood levels of these biomarkers. However, there was a significant intergroup difference in blood sCD40L levels observed before and after the procedure. The UA patient group showed a significantly greater increase in the sCD40L level than the SA group (2.9±0.8 vs. -0.1±0.3 ng/mL, p=0.043). Table 5 shows the correlations between the necrotic core content in the target lesion and the biomarker blood levels. Among the biomarkers evaluated, only pre-PCI blood level of neopterin showed a mildly significant correlation with the absolute volume of the necrotic core in the target lesion (r=0.320, p=0.008). Pre- and post-PCI blood levels of sCD40L (r=0.220, p=0.072; r=0.231, p=0.062) and post-PCI blood level of neopterin (r=0.238, p=0.051) also showed trends toward positive correlation with the absolute volume of necrotic core in the target lesion. However, other biomarker levels were not correlated with relative or absolute content of necrotic core.
The major findings of the present study are as follows: 1) The relative content of necrotic core tended to be higher and the relative content of fibro-fatty tissue tended to be lower in patients with unstable angina than in patients with stable angina. 2) Pre- and post-PCI blood levels of biomarkers such as hsCRP, sCD40L, MMP-9, and neopterin did not differ between the SA group and the UA group. However, the increase in the blood level of sCD40L after PCI was significantly higher in UA patients than in SA patients. 3) Among the various biomarkers evaluated, only pre-PCI blood level of neopterin showed a weakly significant correlation with the absolute volume of the necrotic core in the target lesion. Pre- and post-PCI blood levels of sCD40L and post-PCI blood level of neopterin also showed trends toward a positive correlation with the absolute volume of necrotic core in the target lesion.
Inflammation plays a key role in atherosclerosis and plaque vulnerability. CRP is a liver-derived acute phase protein involved in innate immune response.18 CRP has been found to induce the expression of adhesion molecules, such as Vascular Cell Adhesion Molecule-1, Inter-Cellular Adhesion Molecule-1 and E-selectin, as well as monocyte chemo-attractant factor-1.19,20 CRP also increases tissue factor expression in monocyte-macrophages; promotes monocyte chemotaxis and adhesion to endothelial cells; stimulates the release of reactive oxygen species and MMP-1; and promotes oxidized low-density lipoprotein uptake, which results in increased foam cell formation.21 Recently, several studies discovered CRP in coronary and carotid atheroma.22,23 However, a question of whether CRP directly contributes to atherogenesis is still controversial. Activated platelets express CD40 ligand, which is then cleaved and released as sCD40L.24 The sCD40L binds to circulating monocytes through both its receptor, CD40, and through the monocyte/macrophage integrin Mac-1, and this binding promotes monocyte adhesion to the vascular endothelium.25 The sCD40L also binds to CD40 on endothelial cell surfaces, which activates the expression of adhesion molecules on endothelial cell surfaces.26 MMPs are a family of more than 20 zinc-containing endoproteinases that contribute to collagen degradation and weakening of the fibrous cap.27 Increased expressions of MMP-1, -3 and -9 have been found at the rupture prone shoulder regions of atherosclerotic plaques.28 Neopterin is also produced by activated macrophages and is thought to be a marker of immune activation and macrophage activity.29 Neopterin can act as a pro-oxidant, enhancing oxidant production and promoting cell death or atherosclerotic plaque instability.29 Avanzas, et al.30 reported that serum neopterin is an independent predictor of major adverse coronary events in patients with chronic stable angina pectoris, and Zouridakis, et al.31 also demonstrated that increased serum neopterin levels were associated with the rapid progression of coronary artery disease. The blood levels of these biomarkers are reported to be increased in patients with ACS.32,33 However, only a few in vivo studies have investigated the relationship between estimates of tissue composition determined by VH and periprocedural blood levels of biomarkers. Kubo, et al.34 demonstrated a weak but positive correlation between hsCRP and the amount of necrotic core in the target lesion, and Otake, et al.35 also found a positive correlation between serum hsCRP and the necrotic core ratio and an inverse relationship between serum adiponectin and the necrotic core ratio in both culprit and non-culprit lesions in patients with ACS. Park, et al.36 measured plasma levels of MMP-2 and -9, tissue inhibitor of MMP-1, adiponectin, and macrophage migration inhibitory factor in patients with coronary artery disease and found that only the MMP-9 level showed a weak correlation with the total number of ruptured plaques in both target and non-target vessels. In the present study, we found only a weak correlation between pre-PCI blood level of neopterin and necrotic volume in the PCI target lesion. We also observed that the increase in the blood level of sCD40L after coronary stenting was significantly higher in UA patients than in SA patients. Previous studies have shown that blood levels of inflammatory biomarker rise after PCI possibly due to vascular and myocardial injury related to the procedure.37,38 Kozinski, et al.39 also reported that the rise in the serum levels of hsCRP and serum amyloid A after coronary intervention was greater for UA compared with SA. Although the clinical implication of elevated inflammatory biomarkers after coronary stenting is not fully clarified, a correlation between the levels of CRP after coronary stenting and the cardiovascular event rate during follow-up has been suggested.37,40
There are several potential explanations for weak or poor correlations between the biomarker levels and the relative content of necrotic core by VH in our study. First, our study sample may have been too small to detect any significant relationships between tissue composition analyzed by VH and blood biomarker levels. Second, the blood levels of biomarkers generally represent systemic inflammation and may not reflect local inflammation status or tissue characteristics of PCI target lesions. For instance, we cannot rule out the possibility that the blood level of biomarkers may have been more affected by non-PCI target lesion with mild-to-moderate plaque burden and/or non-coronary atherosclerotic lesions than by the PCI target lesions. Furthermore, non-vascular origins of inflammation may have been responsible for the status of systemic inflammation, although we excluded patients with known infectious or inflammatory disease. Third, potential impact of previous use of statin on the results of the present study cannot be ruled out. Statins are known to reduce blood levels of inflammatory biomarkers and to exert effects on vascular remodeling and plaque composition.41,42 However, whether statins had different degree of influence on blood levels of biomarkers and tissue composition of target lesions in our study cannot be clarified within the limits of the present study. Fourth, tissue characterization by VH may not be sufficient. VH validation studies on human coronary arteries ex vivo have focused solely on the detection of particular tissue types in limited regions of interest. However, VH has never been validated for determination of the absolute or relative areas of any particular tissue component in human coronary arteries. Recently, Thim, et al.43 reported that the necrotic cores of minipig coronary artery plaques assessed by VH did not correlate with pathologic findings, especially when calcifications in the necrotic core were not present or not detected. Therefore, VH may require more pathologic validation to support its reliability.
In conclusion, we found a weak correlation between the pre-PCI neopterin level and necrotic core volume in the PCI-target lesion. The clinical implications of our findings need to be investigated in further studies.
Figures and Tables
ACKNOWLEDGEMENTS
This study was supported partly by grants from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (No. A085012, A102064, and A110879); the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (No.A085136); Yonsei University (6-2009-0008); Korea Institute of Medicine; and the Cardiovascular Research Center, Seoul, Korea.
References
1. Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010. 30:1282–1292.

2. Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997. 336:1276–1282.

3. Libby P, Schoenbeck U, Mach F, Selwyn AP, Ganz P. Current concepts in cardiovascular pathology: the role of LDL cholesterol in plaque rupture and stabilization. Am J Med. 1998. 104:14S–18S.

4. Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, et al. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995. 92:1565–1569.
5. Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994. 90:775–778.

7. Yeh ET, Anderson HV, Pasceri V, Willerson JT. C-reactive protein: linking inflammation to cardiovascular complications. Circulation. 2001. 104:974–975.
8. Burke AP, Tracy RP, Kolodgie F, Malcom GT, Zieske A, Kutys R, et al. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002. 105:2019–2023.

9. Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, et al. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol. 1998. 32:368–372.

10. Gupta S, Fredericks S, Schwartzman RA, Holt DW, Kaski JC. Serum neopterin in acute coronary syndromes. Lancet. 1997. 349:1252–1253.

11. Antoniades C, Bakogiannis C, Tousoulis D, Antonopoulos AS, Stefanadis C. The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J Am Coll Cardiol. 2009. 54:669–677.
12. Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002. 106:2200–2206.

13. Nasu K, Tsuchikane E, Katoh O, Vince DG, Virmani R, Surmely JF, et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006. 47:2405–2412.
14. Hong MK, Mintz GS, Lee CW, Suh J, Kim JH, Park DW, et al. Comparison of virtual histology to intravascular ultrasound of culprit coronary lesions in acute coronary syndrome and target coronary lesions in stable angina pectoris. Am J Cardiol. 2007. 100:953–959.

15. Kawaguchi R, Oshima S, Jingu M, Tsurugaya H, Toyama T, Hoshizaki H, et al. Usefulness of virtual histology intravascular ultrasound to predict distal embolization for ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2007. 50:1641–1646.

16. Kawamoto T, Okura H, Koyama Y, Toda I, Taguchi H, Tamita K, et al. The relationship between coronary plaque characteristics and small embolic particles during coronary stent implantation. J Am Coll Cardiol. 2007. 50:1635–1640.

17. Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001. 37:1478–1492.

18. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003. 111:1805–1812.

19. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000. 102:2165–2168.

20. Pasceri V, Cheng JS, Willerson JT, Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001. 103:2531–2534.

21. Jialal I, Devaraj S, Venugopal SK. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension. 2004. 44:6–11.

22. Yasojima K, Schwab C, McGeer EG, McGeer PL. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am J Pathol. 2001. 158:1039–1051.

23. Kobayashi S, Inoue N, Ohashi Y, Terashima M, Matsui K, Mori T, et al. Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of C-reactive protein. Arterioscler Thromb Vasc Biol. 2003. 23:1398–1404.

24. Hermann A, Rauch BH, Braun M, Schrör K, Weber AA. Platelet CD40 ligand (CD40L)--subcellular localization, regulation of expression, and inhibition by clopidogrel. Platelets. 2001. 12:74–82.

25. Zirlik A, Maier C, Gerdes N, MacFarlane L, Soosairajah J, Bavendiek U, et al. CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation. 2007. 115:1571–1580.

27. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006. 69:562–573.

28. Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994. 94:2493–2503.

29. Gieseg SP, Crone EM, Flavall EA, Amit Z. Potential to inhibit growth of atherosclerotic plaque development through modulation of macrophage neopterin/7,8-dihydroneopterin synthesis. Br J Pharmacol. 2008. 153:627–635.

30. Avanzas P, Arroyo-Espliguero R, Quiles J, Roy D, Kaski JC. Elevated serum neopterin predicts future adverse cardiac events in patients with chronic stable angina pectoris. Eur Heart J. 2005. 26:457–463.

31. Zouridakis E, Avanzas P, Arroyo-Espliguero R, Fredericks S, Kaski JC. Markers of inflammation and rapid coronary artery disease progression in patients with stable angina pectoris. Circulation. 2004. 110:1747–1753.

32. Prasad A, Tsimikas S. Candidate biomarkers for the detection of coronary plaque destabilization and rupture. Curr Atheroscler Rep. 2008. 10:309–317.

33. Naruko T, Furukawa A, Yunoki K, Komatsu R, Nakagawa M, Matsumura Y, et al. Increased expression and plasma levels of myeloperoxidase are closely related to the presence of angiographically-detected complex lesion morphology in unstable angina. Heart. 2010. 96:1716–1722.

34. Kubo T, Matsuo Y, Hayashi Y, Yamano T, Tanimoto T, Ino Y, et al. High-sensitivity C-reactive protein and plaque composition in patients with stable angina pectoris: a virtual histology intravascular ultrasound study. Coron Artery Dis. 2009. 20:531–535.

35. Otake H, Shite J, Shinke T, Watanabe S, Tanino Y, Ogasawara D, et al. Relation between plasma adiponectin, high-sensitivity C-reactive protein, and coronary plaque components in patients with acute coronary syndrome. Am J Cardiol. 2008. 101:1–7.

36. Park JP, Lee BK, Shim JM, Kim SH, Lee CW, Kang DH, et al. Relationship between multiple plasma biomarkers and vulnerable plaque determined by virtual histology intravascular ultrasound. Circ J. 2010. 74:332–336.

37. Gottsauner-Wolf M, Zasmeta G, Hornykewycz S, Nikfardjam M, Stepan E, Wexberg P, et al. Plasma levels of C-reactive protein after coronary stent implantation. Eur Heart J. 2000. 21:1152–1158.

38. Kim JY, Ko YG, Shim CY, Park S, Hwang KC, Choi D, et al. Comparison of effects of drug-eluting stents versus bare metal stents on plasma C-reactive protein levels. Am J Cardiol. 2005. 96:1384–1388.

39. Kozinski M, Krzewina-Kowalska A, Kubica J, Zbikowska-Gotz M, Dymek G, Piasecki R, et al. Percutaneous coronary intervention triggers a systemic inflammatory response in patients treated for in-stent restenosis -- comparison with stable and unstable angina. Inflamm Res. 2005. 54:187–193.

40. Azar RR, McKay RG, Kiernan FJ, Seecharran B, Feng YJ, Fram DB, et al. Coronary angioplasty induces a systemic inflammatory response. Am J Cardiol. 1997. 80:1476–1478.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download