Abstract
Purpose
This study aimed to estimate the prevalence and incidence of cerebrovascular disease (CVD) and stroke in Korean male adolescents.
Materials and Methods
The authors reviewed all medical certificates, medical records, and radiologic images from the examinations of Korean military conscription from January 2008 to May 2011.
Results
Of the 101156 examinees, 40 had CVD and stroke during adolescence. The overall prevalence and incidence of CVD and stroke was 39.54 cases per 100000 adolescents and 2.08 cases per 100000 adolescents per year, respectively and these were similar to the worldwide data. There were 3 cases of aneurysm, 3 cases of dural arteriovenous fistula, 11 cases of arteriovenous malformation, 4 cases of cavernous hemangioma, 4 cases of cerebrovascular infarction, 16 cases of Moyamoya disease, and 1 case of missing data. The incidence of arteriovenous malformation (0.57 cases per 100000 adolescents per year) was lower than the incidence for the worldwide general population. The incidence of Moyamoya disease was higher than that in any other country (15.82 cases per 100000 adolescents, vs. 0.83 cases per 100000 adolescents per year).
With a worldwide incidence of 1.3 to 13 per 100000 adolescents per year, cerebrovascular disease (CVD) and stroke has become an important cause of morbidity and mortality in adolescents; it is already one of the top ten causes of death in adolescents.1-4 The incidence has increased due to increased recognition, less invasive vascular diagnostics [magnetic resonance images (MRI), magnetic resonance angiography or computed tomography (CT) angiography], and therapeutic advances which allow adolescents with predisposing conditions to survive.
Clinically, CVD and stroke present as either hemorrhagic or ischemic, but clinicians may also find incidentally. Hemorrhagic presentations include intracerebral hemorrhage (the commonest form in children under 10) and subarachnoid hemorrhage (the commonest form in children and adolescents over 10).5 These clinical presentations in adolescents are usually caused by arteriovenous malformation (AVM), cerebral cavernous malformation (CCM), cerebral aneurysm, venous sinus thrombosis (VST), or Moyamoya disease (MMD). Ischemic stroke is commonly arterial ischemic stroke (AIS) or cerebral VST. The underlying cause or trigger may be a diagnostic clue to the distinction between AIS, VST and stroke mimics.6-11 Adolescent CVD and stroke are strongly associated with death critically, and also with disability.12 Unfortunately, however, there have been few epidemiologic studies on CVD and stroke in Korean adolescents. Only a few studies have reported some of ischemic stroke's characteristics in Korean young adults compared to the number of western studies.13 Herein, we reviewed 101156 examinees (examined for conscription into the Korean military) to estimate prevalence and incidence of the cerebrovascular disease and stroke in the Korean male adolescent population.
Korea has a conscription system, and all men at age 19 are examined for the purposes of conscription at the Military Manpower Administration. We conducted this cross-sectional survey at the Korean Military Manpower Administration in Seoul, from January 2008 to May 2011, with the approval of the Military Manpower Administration Committee. There were 101156 examinees, aged 19 to 20 years. During this period, 45 men had medical records of CVD and stroke, and 5 cases were excluded from this study because the diagnosis age exceeded 19 years old. We retroactively reviewed all medical records and radiographic images (skull radiographs and CT, MRI, or MR angiographies) of CVD and stroke. Most such cases were rechecked by CT or MRI angiography. And the data were categorized to 6 groups; aneurysm, dural arteriovenous fistula, arteriovenous malformation, cavernous hemangioma, cerebrovascular infarction, and Moyamoya disease. Total and each estimated prevalence and incidence were calculated.
The prevalence of CVD and stroke in a population is defined as the total number of cases in the population (total conscription examinees aged 19 to 20), divided by the number of individuals in the population (total conscription examinees aged 19 to 20). The incidence rate is the number of new cases per population in a given time period, when the denominator is the sum of the person-time of at risk population. It's reasonable to include the mortality and morbidity cases in this study; these cases could not be investigated because the data were derived from the military conscription.
The statistical significances of differences were calculated via Chi-square tests. p-values less than 0.05 were regarded as statistically significant. Statistical comparisons were analyzed with SPSS 12.0 (SPSS Inc., Chicago, IL, USA).
In this study, 45 men had medical records of CVD and stroke, and 5 cases were excluded in this study because the diagnosis age exceeded 19 years old (1 cases of aneurysm with 22 years old of diagnostic age, 2 cases of AVM with 20 and 22 years old, 2 cases of cavernous malformation with 20 and 22 year old). Therefore, 40 cases (0.04%) of 101156 conscription examinees in Korea received diagnoses of CVD and stroke under the age of 19. Among them, we could not find any of medical records and image studies in 1 case. Nevertheless, his medical certificate showed that the examinee did have CVD, and we could not categorize the result to any the type of CVD. The overall prevalence and incidence rate of CVD and stroke was 39.54 and 2.08 cases per 100000 adolescent per year, respectively. There were 3 cases of aneurysm, 3 cases of dural arteriovenous fistula, 11 cases of arteriovenous malformation, 4 cases of cavernous hemangioma, 4 cases of cerebrovascular infarction, 16 cases of Moyamoya disease, and 1 case of missing data. Two cases of aneurysm coexisted with dural arteriovenous fistula or Moyamoya disease.
This study found 3 cases of cerebral aneurysm, representing a prevalence of 2.97 cases per 100000 adolescents, or an incidence of 0.16 cases per 100000 adolescents per year, in Korean male adolescents. Table 1 shows clinical information on the cerebral aneurysms. There were 2 cases combined with other cerebrovascular diseases, such as dural arteriovenous fistula (DAVF) or MMD. A case with a ruptured pseudoaneurysm presented with mental changes. In 1 case of ruptured pseudoaneurysm, clinicians observed vasospasm in the left distal internal cerebral artery, left proximal middle cerebral artery (M1), and proximal left anterior cerebral artery (A1). We did not observe the multiple aneurysm cases in this study.
We also found 3 cases of DAVF, representing a prevalence of 2.97 cases per 100000 adolescents and an incidence of 0.16 cases per 100000 adolescents per year. Table 2 shows clinical information on the DAVFs. One case presented with right exophthalmos with 6th nerve palsy after penetration of by a wooden chopstick at age 5. In conventional cerebral angiography and CT, its feeder appeared as the right carotid artery, and it drained into the ipsilateral orbit vein. Another case presented with general tonic type seizures at age 6, without any definite trauma history. It had a feeder in the right posterior cerebral artery, and it drained into the vein of Galen and right transverse sinus. The last case presented with right periorbital ecchymosis ("raccoon eye") after a traffic accident. In conventional cerebral angiography, its feeder appeared as the right middle meningeal artery, and it drained into the right sphenoparietal sinus. Incidentally, we found that this case also had a pseudoaneurysm of the middle meningeal artery. All cases were treated by embolization. In the DAVF case with pseudoaneurysm, the DAVF recurred 1 year later after embolization and re-embolization was performed.
We found 11 cases of cerebral arteriovenous malformation, giving a prevalence of 10.87 cases per 100000 adolescents and incidence of 0.57 cases per 100000 adolescents per year. Table 3 shows the AVM clinical information. The median age when the AVM symptoms presented was 16 years (range, 6-19 years, with 1 case of missing data). Among the 11 cases, 5 presented with ruptured AVMs (headache, weakness, seizure, and mental changes), and 6 cases presented with unruptured AVMs (headache, seizure, syncope, and memory impairment). Additionally, 6 AVM cases were Spetzler-Martin (SM) grade 2, 2 cases were SM grade 1, and 3 cases were not calculable due to insufficient medical information. With regard to treatment, 3 cases received decompression operations, 4 cases underwent operations for nidus removal, 5 cases received radiotherapy, and 3 cases received embolization. Conventional cerebral angiographies were performed in 7 cases (Table 4). However, we could not be certain whether the other 4 cases underwent conventional cerebral angiography. The records showed that 4 AVM cases experienced significant complications: 2 cases had visual disturbances, 1 case had memory impairment, and 1 case experienced speech impediment and motor weakness.
We found 4 cases of cavernous hemangioma among the conscription, giving a prevalence of 3.95 cases per 100000 adolescents and an incidence of 0.21 cases per 100000 adolescents per year. Table 4 shows clinical information. Presentation symptoms were weakness and seizure. Of these cases, 3 CCMs were located on the subcortical area, and 1 was on the insular. Recurrent bleeding was observed in all these CCMs.
In this study, 4 cases of cerebovascular infarction was found, and the prevalence and incidence rate were 3.95 cases per 100000 adolescents, or an incidence of 0.21 cases per 100000 adolescents per year. Table 5 shows clinical information about the cerebovascular infarction. Presenting symptoms were weakness in 3 cases and disequilibrium in 1 case. The infarcted lesions were randomly distributed as 2 cases of MCA territory, 1 cases of centrum semioval, and 1 cases of cerebellum. All cases were induced by arterial ischemic stroke. Other systemic review was not presented by the medical records.
Finally, we found 16 cases of MMD, giving a prevalence of 15.82 cases per 100000 adolescents and an incidence of 0.83 cases per 100000 adolescents per year. Table 6 shows MMD clinical information. The median age when the symptoms presented was 11 years (range, 8-18 years). Among these 16 cases, 15 (94%) had initial symptoms caused by decreased cerebral perfusion (transient ischemic attack, dysarthria, and seizure), and 1 case presented with mental changes due to intraventricular hemorrhage from the Moyamoya vessels. In review of image study, the brain parenchymal ischemic encephalomalatic change was observed in 9 cases (60%, among 15 cases confirmed by image study, 1 case with missed image data) (Table 6). All cases had been operated [enecephaloduroarteriosynangiogenesis (EDAS), enecephaloduroarteriomyosynangiogenesis (EDAMS), extra-intracranial arterial bypass (EIAB), or combined operations]. We observed 30 MMD locations among these cases; Suzuki grade 4 was in 9 locations, and Suzuki grades 3 and below were in 21 locations. During the imaging studies of the conscription, clinicians observed postoperative encephalomalatic changes in 9 cases and 11 locations (37%, we could not check 1 case's imaging study from the conscription data). The encephalomalatic changes were found mostly in the frontal lobe. This change did not correlate with the Suzuki grade (p-value=0.722). One case was diagnosed with MMD with pseudoaneurysm (Table 6). This 2 mm pseudoaneurysm was in the proximal segment of the left anterior cerebral artery (A1 segment). In this case, the MMD was treated with EDAS and the pseudoaneurysm with observation. The medical records did not indicate whether pseudoaneurysm's disappearance was checked after treatment for MMD.
Cerebrovascular disease and stroke have been important causes of morbidity and mortality in adolescents; they are already among the top ten causes of death in adolescents.1-4 Hemorrhagic stroke has been reported to be more common than ischemic stroke in children and young adults in the United States,3,14,15 however, more recent studies have found different results. Two large hospital discharge studies in the United States reported higher rates of ischemic than hemorrhagic stroke,16,17 and a study of a California-wide hospital discharge database found an incidence rate of 1.1 cases per 100000 person-years for hemorrhagic stroke and 1.2 cases per 100000 person-years for ischemic stroke. These rates and proportions are similar to those in the present study.
In this study, the prevalence of CVD and stroke was 39.54 cases per 100000 adolescents, or an incidence of 2.08 cases per 100000 adolescents per year, respectively. The incidence of hemorrhagic stroke was 1.09 cases per 100000 adolescents per year (3 cases of aneurysm, 3 DAVFs, 11 AVMs, and 4 CCMs were included in the hemorrhagic stroke) and the incidence of ischemic stroke was 1.04 cases per 100000 adolescents per year (4 cases of cerebral infarction, and 16 Moyamoya diseases were included in the ischemic stroke). Thus, nearly a half of adolescent CVD and stroke was hemorrhagic or ischemic stroke. Regarding hemorrhagic stroke, the incidence worldwide is shown in Table 7. The incidence and proportion of aneurysm, AVM, and CCM are not significantly different to worldwide data.
Intracranial aneurysms in adolescents are rare, most studies quoting an incidence of 5% or less.26 In one cooperative study, there was only one child less than 4 years of age with an intracranial aneurysm.27 In the 1000-human cadaver study, just one person under 20 years old harbored an intracranial aneurysm.28 Nevertheless, the actual incidence is not yet clear in the literature review. Generally, females are more common than males, but the study about gender predilection among adolescent is rare.29 In a recent study, simple prevalence and incidence proportions of cerebral aneurysms in adolescents were found to be 2.97 cases per 100000 adolescents and 0.16 cases per 100000 adolescents per year.
In this study, we found only one case of an actual, true-type aneurysm in a person under 20 years old, and the other cases were combined with other diseases such as DAVF or MMD. Aneurysms with DAVF or MMD may arise because of hemodynamic stress. The combination of aneurysm and DAVF is not well known, but aneurysm with MMD or AVM is not uncommon. In one report, 3 cases among 40 patients (7.5%) had an accompanying aneurysm.30 Aneurysm with MMD tends to occur in the posterior circulation. The most commonly involved location seems to be the basilar bifurcation. However, in this study, we found an aneurysm with MMD in the proximal anterior cerebral artery (A1). Generally, the incidence of multiple aneurysms appears low, and we observed no such cases in this study.31,32
The DAVF prevalence is unknown, as some DAVFs remain asymptomatic for years. The estimated incidence is 0.17 per 100000 persons, but this is likely underestimated, because of number of asymptomatic, unreported lesions.33 We found 3 cases of DAVF in the conscription in Korea. This incidence is similar to that in the general population, but it may not be exact because of underestimation among our examinees. No gender predilection was reported.29
Cerebral AVMs constitute a complex tangle of abnormal arteries and veins. The anatomical absence of a capillary bed in the AVM nidus leads to high-flow arteriovenous shunting through one or more fistulas.34 Generally, the AVM detection rate is 1.11-1.21 per 100000 people per year, and the incidence of AVM hemorrhage is 0.42 per 100000 people per year.33,35 In our literature review, we found scant data on AVMs among adolescents. In this study, we found 11 AVM cases (10.87 cases per 100000 adolescents, or 0.57 cases per 100000 adolescents per year) and also found that the incidence in Korean adolescents was lower than the general, worldwide incidence. No gender predilection was reported in the literature.29
In AVMs that present with hemorrhage, the rate for first hemorrhage is 2-4% and the rate for recurrent hemorrhage is 6-18% in the initial year, declining to pre-hemorrhage rate over 5 years.33 Kondziolka, et al. approximated the chances of an AVM hemorrhage according to age by an equation: Risk of hemorrhage=1 - (risk of no hemorrhage)expected years of remaining life.36 In a group of 19-year-olds the approximate 19-year-olds' risk of hemorrhage is 64%, if the expected years of remaining life is 60, and the risk of hemorrhage is 1.7% per year. In this study, we found 11 AVM cases, of which 5 cases had ruptured AVM (45%), ant that all well-formed AVMs were SM grades 1 or 2. The recommended general treatment strategy for SM grades 1 and 2 AVMs is surgery or focused irradiation. In this review, 6 among the 8 cases of known SM grade 1 or 2 received surgical treatment and/or radiosurgery, and 2 cases received surgical treatment or radiosurgery after embolization.
The exact incidence and prevalence of CCMs are unknown, as many CCMs are asymptomatic. A population-based study showed an incidence of 0.15 per 100000 persons per year.33 In some autopsy and MRI studies, the prevalence was 0.4-0.9%.37-39 No gender predilection was reported.29 In this study, we found 4 cases of CCM, giving a prevalence of 3.95 cases per 100000 adolescents and an incidence of 0.21 cases per 100000 adolescents per year. Generally, evidence of previous hemorrhage is present at every lesion, regardless of clinical history. In addition, CCMs have persistent intralesional microhemorrhages, which occur over time. We found such previous hemorrhages in all CCM cases in this study.
Adolescent ischemic stroke is not as rare as one might surmise. Incidence estimates vary, depending on definition and inclusion criteria. AIS ranges from 0.6 to 7.9 per 100000 adolescents under age 18 per year in North America and Europe.3,4 Arterial ischemic stroke is defined as an acute focal neurological deficit lasting longer than 24 hours with neuroimaging evidence of cerebral infarction. There is no gender predilection in cerebrovascular infarction, but AIS is more common in male than female.29 Arterial ischemic stroke is a complex disease with many risk factors. The most frequently reported risk factors for AIS in adolescent are cardiac disorders, hematological disorders, metabolic disorders, arteriopathies, and infection.40,41 Unfortunately, however, systemic review of risk factors was not investigated in this study. Total 4 cases of cerebovascular infarction by AIS were found in this study, and the prevalence and incidence rate was 3.95 cases per 100000 adolescents, or an incidence of 0.21 cases per 100000 adolescents per year, respectively. The incidence is lower than that in any other reports, but similar to that including Moyamoya disease (other reports included Moyamoya disease to AIS as arteriopathies).
Moyamoya, meaning a "hazy puff of smoke" in Japanese, is a chronic, occlusive cerebrovascular disease involving bilateral stenosis or occlusion of the terminal portion of the internal carotid arteries and/or the proximal portions of the anterior cerebral arteries and middle cerebral arteries.42 The MMD incidence is high in East Asian countries, such as Japan and Korea (Table 8). In Japan, the estimated annual prevalence and incidence are 6.03 and 0.54 per 100000 persons, respectively.43 MMD's clinical background in Korea is essentially similar to that in Japan.44 In our series, we found 16 MMD cases among the young conscripted men in Korea (15.85 cases per 100000 adolescents, 0.83 cases per 100000 adolescents per year). Our literature review revealed that this is the highest prevalence and second-highest incidence, next to the report by Baba, et al. In Korea and Japan, the number of women with MMD is higher than that of men.47 Because we based our present study on the examination of conscripts, we did not include female adolescents. Therefore, if this epidemiological study extends to all male and female adolescents, the prevalence and incidence proportions would be higher than those shown in this study.
In this study, mean age at diagnosis was 11.5 years (range, 8-18 years), and 15 (93.5%) of 13 MMD cases presented with decreased perfusion; 1 case presented with hemorrhage by MMD. Clinically, young patients with MMD present with ischemic attacks, and adult patients present with either ischemic or hemorrhagic events.45 Although this point has recently been debated. Generally, most patients present with bilateral involvement; up to 18% of patients have angiographically documented unilateral involvement.46 In this study, we observed unilateral involvement in 2 (12.5%) of 16 cases. In addition, 1 case had a pseudoaneurysm, located in the proximal anterior cerebral artery (A1).
This study was based on the data of Korean military conscription. Therefore, a few limitations in the epidemiologic study were present. First, pass away or severely disabled examinees were excluded from the conscription, so mortality or morbidity cases were not included in this study. Nevertheless, according to the data of Seoul regional Military Manpower Administration, less than 0.01% of cases were excluded from the conscription by severe disability, and these morbidity cases do not seem to affect the result. Second, only male adolescent was included in this study. Thus, overall prevalence and incidence calculated could underappreciate or not indicate the general population rate by different gender factors. The authors used reference data to supplement this problem, however, it was not enough. The general population study would be needed to this problem. Finally, by using medical certificate and record, not all conscription cases were checked for image study. The general symptom of CVD and stroke is headache and other popular symptom, therefore, undiagnosed incidental CVD and stroke cases could be present. However, this limitation was applicable not only to this study, but also to all epidemiologic study. To solve this limitation, well designed large population study would be needed; also it is very difficult.
Regardless of limitations, this study is a large cross-sectional survey with 101156 examinees with localized geographical distribution, same ethics, and specific 19 years old aged males. Consequently, this study is very useful for estimating the prevalence rates and types of CVD in Korean male adolescents.
In conclusion, in Korean male adolescents, the incidence of cerebrovascular disease and stroke (2.08 cases per 100000 adolescents per year) and the proportions of hemorrhagic and ischemic stroke are similar to those worldwide. However, the incidence of arteriovenous malformation (0.57 cases per 100000 adolescents per year) was lower than the incidence of worldwide, general population. The incidence of Moyamoya disease was higher than that in any other country (for a prevalence of 15.82 cases per 100000 adolescents and an incidence of 0.83 cases per 100000 adolescents per year).
Figures and Tables
Table 6
The Clinical Information of 16 Moyamoya Disease
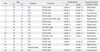
Age of Ex, the age with the examination for conscription; Age of Dx, the age with the diagnosis; EDAS, enecephaloduroarteriosynangiogenesis; EDAMS, enecephaloduroarteriomyosynangiogenesis; EIAB, extra-intracranial arterial bypass; TIA, transient ischemic attack.
*A case of Moyamoya disease with pseudoaneurysm.
†Symptom was developed by the intraventricular hemorrhage.
ACKNOWLEDGEMENTS
This study was approved by Seoul Regional Military Manpower Administration. Special thanks to Beom-kyu Lee, Yong-taek Kim, and Cheol-ho Park in Seoul Regional Military Manpower Administration for help to review the medical records.
References
1. Zahuranec DB, Brown DL, Lisabeth LD, Morgenstern LB. Is it time for a large, collaborative study of pediatric stroke? Stroke. 2005. 36:1825–1829.

2. Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002. 109:116–123.

3. Earley CJ, Kittner SJ, Feeser BR, Gardner J, Epstein A, Wozniak MA, et al. Stroke in children and sickle-cell disease: Baltimore-Washington Cooperative Young Stroke Study. Neurology. 1998. 51:169–176.

4. Giroud M, Lemesle M, Gouyon JB, Nivelon JL, Milan C, Dumas R. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: a study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995. 48:1343–1348.

5. Fullerton HJ, Wu YW, Sidney S, Johnston SC. Recurrent hemorrhagic stroke in children: a population-based cohort study. Stroke. 2007. 38:2658–2662.
6. Kurnik K, Kosch A, Sträter R, Schobess R, Heller C, Nowak-Göttl U. Childhood Stroke Study Group. Recurrent thromboembolism in infants and children suffering from symptomatic neonatal arterial stroke: a prospective follow-up study. Stroke. 2003. 34:2887–2892.

7. Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003. 53:167–173.

8. deVeber G, Andrew M, Adams C, Bjornson B, Booth F, Buckley DJ, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001. 345:417–423.

9. Heller C, Heinecke A, Junker R, Knöfler R, Kosch A, Kurnik K, et al. Cerebral venous thrombosis in children: a multifactorial origin. Circulation. 2003. 108:1362–1367.
10. Sébire G, Tabarki B, Saunders DE, Leroy I, Liesner R, Saint-Martin C, et al. Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain. 2005. 128(Pt 3):477–489.

11. Shellhaas RA, Smith SE, O'Tool E, Licht DJ, Ichord RN. Mimics of childhood stroke: characteristics of a prospective cohort. Pediatrics. 2006. 118:704–709.

13. Kwon SU, Kim JS, Lee JH, Lee MC. Ischemic stroke in Korean young adults. Acta Neurol Scand. 2000. 101:19–24.

14. Schoenberg BS, Mellinger JF, Schoenberg DG. Cerebrovascular disease in infants and children: a study of incidence, clinical features, and survival. Neurology. 1978. 28:763–768.

15. Broderick J, Talbot GT, Prenger E, Leach A, Brott T. Stroke in children within a major metropolitan area: the surprising importance of intracerebral hemorrhage. J Child Neurol. 1993. 8:250–255.

16. Lynch JK. The hospitalization of childhood stroke in the United States, 1979-2000 [poster presentation, abstract]. Stroke. 2003. 34:287.
17. Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003. 61:189–194.

18. Eeg-Olofsson O, Ringheim Y. Stroke in children. Clinical characteristics and prognosis. Acta Paediatr Scand. 1983. 72:391–395.

19. Visudhiphan P, Chiemchanya S, Wattanasirichaigoon D. Strokes in Thai children: etiology and outcome. Southeast Asian J Trop Med Public Health. 1996. 27:801–805.
20. Giroud M, Lemesle M, Madinier G, Manceau E, Osseby GV, Dumas R. Stroke in children under 16 years of age. Clinical and etiological difference with adults. Acta Neurol Scand. 1997. 96:401–406.

21. Lin CL, Loh JK, Kwan AL, Howng SL. Spontaneous intracerebral hemorrhage in children. Kaohsiung J Med Sci. 1999. 15:146–151.
22. Lanthier S, Carmant L, David M, Larbrisseau A, de Veber G. Stroke in children: the coexistence of multiple risk factors predicts poor outcome. Neurology. 2000. 54:371–378.

23. Blom I, De Schryver EL, Kappelle LJ, Rinkel GJ, Jennekens-Schinkel A, Peters AC. Prognosis of haemorrhagic stroke in childhood: a long-term follow-up study. Dev Med Child Neurol. 2003. 45:233–239.

24. Meyer-Heim AD, Boltshauser E. Spontaneous intracranial haemorrhage in children: aetiology, presentation and outcome. Brain Dev. 2003. 25:416–421.

25. Liu AC, Segaren N, Cox TS, Hayward RD, Chong WK, Ganesan V, et al. Is there a role for magnetic resonance imaging in the evaluation of non-traumatic intraparenchymal haemorrhage in children? Pediatr Radiol. 2006. 36:940–946.

27. Wani AA, Phadke R, Behari S, Sahu R, Jaiswal A, Jain V. Role of diffusion-weighted MRG in predicting outcome in subarachnoid hemorrhage due to anterior communicating artery aneurysms. Turk Neurosurg. 2008. 18:10–16.
28. Kapoor K, Kak VK. Incidence of intracranial aneurysms in north-west Indian population. Neurol India. 2003. 51:22–26.
29. Osborn AG, Blaser SI, Salzman KL, Katzman GL, Provenzale J, Castillo M. Diagnostic imaging brain. 2004. Salt Lake City (Utah): Amirsys, Inc.
30. Kwon TH, Moon SH, Lim DJ, Park YK, Chung HS, Lee HK, et al. Intracranial aneurysm associated with moyamoya disease. J Korean Neurosurg Soc. 1999. 28:1661–1665.
31. Hourihan MD, Gates PC, McAllister VL. Subarachnoid hemorrhage in childhood and adolescence. J Neurosurg. 1984. 60:1163–1166.

32. Meyer FB, Sundt TM Jr, Fode NC, Morgan MK, Forbes GS, Mellinger JF. Cerebral aneurysms in childhood and adolescence. J Neurosurg. 1989. 70:420–425.

33. Brown RD Jr, Wiebers DO, Torner JC, O'Fallon WM. Frequency of intracranial hemorrhage as a presenting symptom and subtype analysis: a population-based study of intracranial vascular malformations in Olmsted Country, Minnesota. J Neurosurg. 1996. 85:29–32.

34. Mohe JP, Pile-Spellman J, Stein BM. Barnett JM, Mohr JP, Stein BM. Arteriovenous malformations and other vascular anomalies. Stroke, Pathophysiology, Diagnosis, and Management. 1998. 3rd ed. Philadephia: Churchill Livingstone;725–750.
35. Stapf C, Mohr JP, Pile-Spellman J, Solomon RA, Sacco RL, Connolly ES Jr. Epidemiology and natural history of arteriovenous malformations. Neurosurg Focus. 2001. 11:e1.

36. Kondziolka D, McLaughlin MR, Kestle JR. Simple risk predictions for arteriovenous malformation hemorrhage. Neurosurgery. 1995. 37:851–855.

37. Otten P, Pizzolato GP, Rilliet B, Berney J. [131 cases of cavernous angioma (cavernomas) of the CNS, discovered by retrospective analysis of 24,535 autopsies]. Neurochirurgie. 1989. 35:82–83. 128–131.
38. Kim DS, Park YG, Choi JU, Chung SS, Lee KC. An analysis of the natural history of cavernous malformations. Surg Neurol. 1997. 48:9–17.

39. Sage MR, Brophy BP, Sweeney C, Phipps S, Perrett LV, Sandhu A, et al. Cavernous haemangiomas (angiomas) of the brain: clinically significant lesions. Australas Radiol. 1993. 37:147–155.

40. Sträter R, Becker S, von Eckardstein A, Heinecke A, Gutsche S, Junker R, et al. Prospective assessment of risk factors for recurrent stroke during childhood--a 5-year follow-up study. Lancet. 2002. 360:1540–1545.

41. Kirkham FJ, Prengler M, Hewes DK, Ganesan V. Risk factors for arterial ischemic stroke in children. J Child Neurol. 2000. 15:299–307.

42. Suzuki J, Takaku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969. 20:288–299.
43. Kuriyama S, Kusaka Y, Fujimura M, Wakai K, Tamakoshi A, Hashimoto S, et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. 2008. 39:42–47.

44. Ikezaki K, Han DH, Kawano T, Kinukawa N, Fukui M. A clinical comparison of definite moyamoya disease between South Korea and Japan. Stroke. 1997. 28:2513–2517.

45. Burke GM, Burke AM, Sherma AK, Hurley MC, Batjer HH, Bendok BR. Moyamoya disease: a summary. Neurosurg Focus. 2009. 26:E11.

46. Kelly ME, Bell-Stephens TE, Marks MP, Do HM, Steinberg GK. Progression of unilateral moyamoya disease: a clinical series. Cerebrovasc Dis. 2006. 22:109–115.

47. Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry. 2008. 79:900–904.

48. Wakai K, Tamakoshi A, Ikezaki K, Fukui M, Kawamura T, Aoki R, et al. Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg. 1997. 99:Suppl 2. S1–S5.

49. Su CF, Shih CJ, Lin LS, Hung TP. [Moyamoya disease in Taiwan]. J Formos Med Assoc. 1994. 93:Suppl 2. S90–S97.
50. Choi SK, Kim GK, Kim HD, Lim YJ, Kim TS, Rhee BA, et al. Natural history of moyamoya disease. J Korean Neurosurg Soc. 1995. 24:536–545.
51. Marcinkevicius E, Liutkus D, Gvazdaitis A. Experience of treatment of moyamoya disease at the Clinic of Neurosurgery of Kaunas University of Medicine. Medicina (Kaunas). 2006. 42:130–136.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download