Abstract
Acute sensorimotor polyneuropathy that resembles Guillain-Barré syndrome (GBS) is rarely accompanied with nephrotic syndrome, and its underlying immunological mechanisms are unclear. A 56-year-old man presented with simultaneous acute progressive symmetric sensorimotor polyneuropathy and proteinuria. A kidney biopsy revealed focal segmental glomerulosclerosis. Serial electrophysiologic studies showed only a transient proximal conduction block in the median nerve, stimulated somatosensory evoked potential and prolonged terminal latencies of the median and peroneal nerves. The patient's neurologic deficits and kidney dysfunction recovered with corticosteroid treatment. Our case showed that somatosensory evoked potential study can be an important objective tool in the diagnosis of acute polyneuropathy with normal distal nerve conduction and that corticosteroids should be considered in the initial treatment of GBS-resembling polyneuropathy associated with nephrotic syndrome.
Nerve conduction study (NCS) is a mainstay in the diagnosis of peripheral neuropathy. However, conventional NCS is limited when the lesion is located in an inaccessible and proximal segment of the nerve.1 Late response and/or somatosensory evoked potential (SSEP) study can be helpful in these instances. In this paper, we describe a patient with acute sensorimotor polyneuropathy that was accompanied with nephrotic syndrome and whose electrophysiological studies showed a transient and proximal sensory conduction defect and prolonged terminal latencies of the motor nerves.
A 56-year-old man developed progressive paresthesia and weakness over a 3-week period. He developed numbness of the fingertips on both hands a month before, which progressed proximally and subsequently involved the feet. One week before admission, he could not elevate his shoulders and felt unsteady when walking. He denied any preceding upper respiratory infection or diarrhea. On neurologic examination, the proximal muscles of the upper and lower extremities were weaker (Medical Research Council grade 4) than those of the distal limbs. Vibration sense was not perceived below the iliac crest and a pinprick sensation was symmetrically decreased below the wrist and ankle. Deep tendon reflexes were not elicited at all. Cranial nerve and cerebellar function were normal.
Motor and sensory nerve conduction studies performed on the left median, ulnar, posterior tibial, and peroneal nerves were normal. F-latencies were all within normal ranges and bilateral H-reflex was not elicited. Electromyography showed a mild degree of positive sharp waves and fibrillation potentials with reduced recruitment of motor unit action potentials in the left deltoid, first dorsal interossei, vastus lateralis, and tibialis anterior muscles. Median nerve SSEP revealed a prominent positive wave followed by a very low amplitude negative potential on the brachial plexus potential (Erb's point) bilaterally. Both cervical (N13) and cortical (N19) waves were barely discernible (Fig. 1A). A magnetic resonance image scan of the cervical spine was normal. Routine urinalysis revealed proteinuria (1.8 g/24 h). Blood chemistry was normal except for hypoalbuminemia (2.9 g/dL), and immunoelectrophoresis did not show any gammopathy. Cerebrospinal fluid examination revealed a white blood cell count of 0 cells/mm3, a protein level of 27.0 mg/dL, and a glucose level of 68 mg/dL. A percutaneous kidney gun biopsy showed focal segmental glomerulosclerosis (FSGS). Vacuolar changes in the glomerular epithelial cells and diffuse effacement of the foot processes were noted with electromicroscopy. Immunofluorescence revealed segmental granular deposition of IgM, C3, as well as the kappa and lambda light chains along the peripheral capillary wall and mesangium. A follow-up nerve conduction study after 2 weeks showed prolonged terminal latencies of the median (4.95 ms) and peroneal (7.05 ms) nerves but normal motor and sensory nerve conduction of the other nerves.
The patient's neurologic deficits were markedly improved after 5 weeks of corticosteroid treatment. Proteinuria disappeared as the patient's neurologic features improved. Terminal latencies of the median and peroneal nerves returned to normal and H-reflexes were obtained bilaterally. F-latencies were shortened. Negative potentials on the bilateral brachial plexus became prominent with normalization of the cervical and cortical potentials (Fig. 1B).
The case presented in this paper raises two interesting points. The patient had an acute sensorimotor polyneuropathy resembling Guillain-Barré syndrome (GBS) and showed transient involvement of the proximal sensory nerve at the level of the plexus and distal motor nerves, which was confirmed with electrophysiological studies. The other interesting point was the association of FSGS and GBS-like polyneuropathy.
Serial examinations of median nerve SSEP demonstrated a conduction defect at the level of the brachial plexus, which was completely recovered after 5 weeks of steroid treatment. A delay of terminal latencies of the motor nerves was also seen when the patient's symptoms progressed. Remarkably, F-latencies were within normal ranges during the disease course.
It is well known that the initial change in the electrodiagnostic features in GBS is a prolongation or loss of F-waves due to immunologic attack that is thought to begin at nerve roots, where there is a relative lack in blood-nerve barrier. Though SSEP has been reported as a useful test for evaluation of proximal sensory nerve lesions, including chronic inflammatory demyelinating polyradiculoneuropathy and chronic immune sensory polyradiculopathy,2,3 there are only a few studies in regards to SSEP findings in patients with GBS. Most of the findings suggest that F-wave is more sensitive than SSEP for the diagnosis of GBS.4-6 Walsh, et al.7 suggested that SSEP had a higher yield than F-wave latency; however, SSEP was only applied in cases where nerve conduction velocities were also slow. Notwithstanding, one study reported on 10 patients with early GBS who showed abnormal SSEP but normal distal nerve conduction.8 Even though they did not conduct F-wave studies, we speculate that F-latencies would have also been delayed in most of these patients, because the maximal motor conduction velocities across the proximal arm and Erb's point were also slow.
Of particular note, FSGS simultaneously occurred with GBS-mimicking polyneuropathies. In fact, nephrotic syndrome associated with acute polyradiculoneuropathy has been recognized previously.9 However, very few cases have been reported since then, and most of the combined nephrotic syndromes consisted of glomerulonephritis or minimal change nephrotic syndrome. Though FSGS is the most common form of nephrotic syndrome, only three cases associated with GBS have been published so far.10-12 Neither intravenous immunoglobulin nor plasmapheresis was effective in treatment thereof; however, corticosteroids with or without immunosuppressant agents could potentially have ameliorated both the polyneuropathy and the FSGS. We cannot assume that the polyneuropathy and FSGS occurred coincidentally or shared the same immunologic mechanism. However, the time relationship between the two diseases and the parallel improvement with corticosteroid treatment, as shown by our case, raises the possibility that they could share the same immunopathological mechanisms.
In conclusion, our case is instructive in that SSEP can be an important objective tool in the diagnosis of acute polyneuropathy with normal distal nerve conduction and that corticosteroids should be considered in the initial treatment of GBS-resembling polyneuropathy associated with FSGS.
Figures and Tables
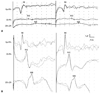 | Fig. 1Serial median nerve SSEP studies using the same filter settings. (A) At admission, the observed increased duration of positive deflection with a low amplitude negative peak of the bilateral brachial plexus (Erb's point) with barely discernible cervical and cortical potentials are shown. (B) Restoration of the waveforms of the SSEP, which was evident 5 weeks after corticosteroid treatment, is shown. SSEP, somatosensory evoked potential. |
References
1. Wilbourn AJ, Aminoff MJ. American Association of Electrodiagnostic Medicine. AAEM minimonograph 32: the electrodiagnostic examination in patients with radiculopathies. Muscle Nerve. 1998. 21:1612–1631.

2. Yiannikas C, Vucic S. Utility of somatosensory evoked potentials in chronic acquired demyelinating neuropathy. Muscle Nerve. 2008. 38:1447–1454.

3. Sinnreich M, Klein CJ, Daube JR, Engelstad J, Spinner RJ, Dyck PJ. Chronic immune sensory polyradiculopathy: a possibly treatable sensory ataxia. Neurology. 2004. 63:1662–1669.

4. Ropper AH, Chiappa KH. Evoked potentials in Guillain-Barré syndrome. Neurology. 1986. 36:587–590.
5. Gilmore RL, Nelson KR. SSEP and F-wave studies in acute inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 1989. 12:538–543.

6. Vajsar J, Taylor MJ, MacMillan LJ, Murphy EG, Logan WJ. Somatosensory evoked potentials and nerve conduction studies in patients with Guillain-Barré syndrome. Brain Dev. 1992. 14:315–318.

7. Walsh JC, Yiannikas C, McLeod JG. Abnormalities of proximal conduction in acute idiopathic polyneuritis: comparison of short latency evoked potentials and F-waves. J Neurol Neurosurg Psychiatry. 1984. 47:197–200.

8. Brown WF, Feasby TE. Sensory evoked potentials in Guillain-Barré polyneuropathy. J Neurol Neurosurg Psychiatry. 1984. 47:288–291.
9. Behan PO, Lowenstein LM, Stilmant M, Sax DS. Landry-Guillain-Barré-Strohl syndrome and immune-complex nephritis. Lancet. 1973. 1:850–854.

10. Heckmann JG, Böhmer K, Druschky A, Winterholler M, Neundörfer B. Guillain-Barré syndrome in association with focal segmental glomerulosclerosis. Eur Neurol. 1998. 40:114–115.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download