Abstract
Purpose
The aim of this prospective, double-blind, randomized study was to investigate the analgesic effects of low-dose ketamine on intravenous patient-controlled analgesia (IV-PCA) with fentanyl for pain control in pediatric patients following the Nuss procedure for pectus excavatum.
Materials and Methods
Sixty pediatric patients undergoing the Nuss procedure were randomly assigned to receive fentanyl (Group F, n=30) or fentanyl plus ketamine (Group FK, n=30). Ten minutes before the end of surgery, following the loading dose of each solution, 0.5 µg/kg/hr of fentanyl or 0.5 µg/kg/hr of fentanyl plus 0.15 mg/kg/hr of ketamine was infused via an IV-PCA pump (basal rate, 1 mL/hr; bolus, 0.5 mL; lock out interval, 30 min). Fentanyl consumption, pain score, ketorolac use, nausea/vomiting, ondansetron use, pruritus, respiratory depression, hallucination, dreaming, and parent satisfaction with pain control were measured throughout the 48 hours following surgery.
Results
The pain scores, ketorolac use, and fentanyl consumption of Group FK were significantly lower than in Group F (p<0.05). The incidence of nausea/vomiting and ondansetron use in Group FK was significantly lower than in Group F (p<0.05). There were no reports of respiratory depression, hallucination or dreaming. Parent satisfaction with pain control was similar between the two groups.
Pectus excavatum is a congenital deformity in which the sternum and the inferior costal cartilage area are depressed. Nuss, et al.1 described the Nuss procedure to correct pectus excavatum using specially made metal rods fitted to the chest deformity of the patient. Bending of the rod pushes against the depressed costa and sternum, thereby restoring the chest to its normal shape. The procedure has excellent esthetic results and helps improve lung function. However, the Nuss procedure can induce severe postsurgical pain due to the expansion of the thorax. This pain can be controlled with intravenous patient-controlled analgesia (IV-PCA) using opioids.2-4
Strong pain stimuli activate N-methyl-D-aspartate (NMDA) receptors and produce hyperexcitability of dorsal root neurons.5 This trigger induces central sensitization, the wind-up phenomenon, and pain memory. An NMDA receptor antagonist can prevent the induction of central sensitization caused by stimulation of peripheral nociception as well as by blocking the wind-up phenomenon.5 When used in a large dose for a prolonged period as well as at the very early stage of therapy, opioids can induce hyperalgesia and tolerance, which may lead to increased postoperative pain.6,7 Increased postoperative pain, in turn, leads to the need for higher doses of opioids. Ketamine, by blocking NMDA receptors, can prevent the development of central sensitization and opioid resistance.5,7 Therein is the basis for using ketamine as an adjuvant to opioids in postoperative pain control.
However, clinical trials of ketamine added to IV-PCA with opioids in postoperative pain control have demonstrated contradictory results.8-11 The effects of ketamine combined with fentanyl in IV-PCA for postoperative pain control for the Nuss procedure have not been examined. Therefore, we investigated the analgesic effects of low-dose ketamine on IV-PCA with fentanyl for pain control following the Nuss procedure for pectus excavatum repair in pediatric patients.
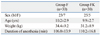
This prospective, double-blind randomized study was approved by the Institutional Review Board. Written informed consent for the study was obtained from the parents of all children enrolled in the study. Sixty children, American Society of Anesthesiologists physical status I or II, aged 6-16 years, who underwent the Nuss procedure for repair of pectus excavatum were enrolled in this study. The patients were divided randomly into two groups. Group F was given fentanyl only, and Group FK was given fentanyl plus ketamine. The equipment and procedure were explained in detail. Patients with congenital heart disease, lung parenchymal disease, gastrointestinal disease, psychiatric disease, skin disease, a history of motion sickness or postoperative nausea/vomiting, drug contraindications, or who were unable to understand how to use the IV-PCA device (AutoMed 3200, Acemedical, Seoul, Korea) were excluded.
One hour prior to the induction of anesthesia, 0.1 mg/kg of atropine was injected intramuscularly as premedication. An IV line was introduced and a dextrose/saline solution was infused at 4 mL/kg/hr during the operation. Anesthesia was induced by 5 mg/kg of thiopental sodium and 0.6 mg/kg of rocuronium. After the trachea was intubated, anesthesia was maintained with 1.5 L/min of oxygen, 1.5 L/min of nitrous oxide and 1.5-2.5 vol% of sevoflurane. Throughout the operation, blood pressure, electrocardiogram, end-tidal carbon dioxide concentration and pulse oximetry saturation were routinely monitored.
Ten minutes before the end of surgery, a loading dose of 1 µg/kg of IV fentanyl or 1 µg/kg of IV fentanyl plus 0.3 mg/kg of IV ketamine was injected for respective each group. Subsequently, 0.5 µg/kg/hr of fentanyl or 0.5 µg/kg/hr of fentanyl plus 0.15 mg/kg/hr of ketamine was infused via an IV-PCA pump with a basal rate of 1 mL/hr respectively to each group. Drug concentrations were prepared for each patient according to his/her body weight, totaling 1 mL of drug preparation comprising 0.5 µg/kg of fentanyl or 0.5 µg/kg of fentanyl plus 0.15 mg/kg of ketamine for each group. When patients experienced pain, they or their guardians pushed the demand button, and, thereafter, a 0.5 mL drug bolus, comprising 0.25 µg/kg of fentanyl or 0.25 µg/kg of fentanyl plus 0.075 mg/kg of ketamine for each respective group, was injected. The frequency of bolus injection was limited to two times per one hour. The total volume of IV-PCA medication was determined to be 100 mL, corresponding to 50 µg/kg of fentanyl or 50 µg/kg of fentanyl plus 15 mg/kg of ketamine for each group, i.e. the maximum dose over 48 hours. The intensity of pain was assessed using a visual analogue scale (VAS) ranging from 0 (no pain) to 10 (worst imaginable pain). VAS and cumulative fentanyl consumption were measured at 1, 6, 12, 24, and 48 hours after the surgery. For patients who responded with pain greater than 4 on the VAS, 0.5 mg/kg of IV ketorolac was administered and the administration was recorded.
A blinded anesthesiologist assessed and recorded the development of side effects including nausea/vomiting, pruritus, respiratory depression, hallucination, and dreaming at 1, 6, 12, 24, and 48 hours after surgery. Nausea was defined as the subjective sensation of discomfort associated with the awareness of the urge to vomit. Vomiting was defined as a forceful expulsion of gastric contents through the mouth. In cases of severe nausea/vomiting, or nausea/vomiting that did not resolve, we administered 0.1 mg/kg of IV ondansetron. Pruritus was defined as the subjective sensation of itching without skin lesions and was treated with 0.1 mg/kg of IV chlorophenyramine or 1 µg/kg of IV naloxone, in severe cases only. Respiratory depression was defined as pulse oximetry saturation levels less than 90% at room air or a respiration rate less than 10/min. Respiratory depression, if present, was managed with oxygen insufflation via nasal cannula at 5 L/min. When respiratory depression was not corrected by oxygen insufflations, 1 µg/kg of IV naloxone was administered. If the respiratory depression was still not corrected by these measures, the IV-PCA infusion rate was to be reduced by 50% every hour, until it was resolved. Respiration rate and pulse oximetry saturation were to be monitored throughout these measures until the respiratory depression was resolved. Hallucination and dreaming were defined as any sensation that was not caused by an external event, and if present, the IV-PCA infusion rate would be reduced by 50% every hour, until symptoms were resolved. Overall parent satisfaction with pain control was determined at 48 hours after surgery as either satisfactory or unsatisfactory.
Michelet, et al.9 hypothesized a 25% reduction in morphine consumption with an expected standard deviation of 30% in a calculated sample size of 24 subjects per group, detecting a difference with a power of 0.80 and a 5% risk of type I error. Based on their study,9 we expected that fentanyl plus ketamine IV-PCA could reduce fentanyl consumption by 25% in a sample size of 24 patients per group with the same statistical power. We planned to enroll enough patients to obtain 30 patients per group. Values were expressed as mean±SD or as the number of patients (%). Statistical analyses were performed using the statistical software package SPSS version 12 for Windows (SPSS Inc., Chicago, IL, USA). For numerical variables, independent t-tests were employed to compare inter-group differences and chi-square or Fisher's exact tests were adopted for categorical variables. Differences were regarded as statistically significant when the p value was less than 0.05.
Sixty five patients were assessed for eligibility. Three patients were not included in the study due to being younger than 6 years of age. Two patients were excluded due to history of motion sickness. In total, sixty patients were enrolled for the study. Thirty patients were randomly allocated to each group (Fig. 1). Gender, age, weight, and duration of anesthesia were not significantly different between the two groups (Table 1).
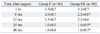
The VAS pain scores of Group FK were significantly lower than those of Group F at 6, 24, and 48 hours after surgery (p<0.05) (Table 2). There were 7 (23%) patients who required additional analgesic, ketorolac, during the 48 hours after surgery, in Group FK, significantly lower than the 18 patients (53%) in Group F (p<0.01).
The cumulative fentanyl consumption was significantly reduced in Group FK compared to Group F, at 24 and 48 hours after surgery (p<0.05) (Table 3).
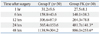
The incidence of nausea/vomiting was 23% in Group FK, significantly lower than 53% in Group F (p<0.05) (Table 4). The number of patients who required the antiemetic, ondansetron, during the 48 hours after surgery was 3 (10%) in Group FK, significantly lower than 11 patients (37%) in Group F (p<0.05). The degree of pruritus was not severe, and the incidence of pruritus was not different between the two groups (Table 4). There were no reports of respiratory depression, hallucination, or dreaming in either group (Table 4). No patients required administration of naloxone or chlorophenyramine. The number of parents satisfied with pain control was 25 (83%) in Group F and 28 (93%) in Group FK, and the difference was not significant.
In our study, administration of IV-PCA with fentanyl plus low-dose ketamine resulted in reductions of the fentanyl consumption and pain scores as well as a decrease in nausea/vomiting over fentanyl alone.
Opioids in IV-PCA are used widely in the management of severe postoperative pain after the Nuss procedure.2-4 Control of severe pain requires more opioids. However, higher doses of opioids are associated with significant side effects including respiratory depression, nausea/vomiting, sedation, constipation, urinary retention, as well as acute tolerance. In our study, we infused a basal rate of 0.5 µg/kg/hr of fentanyl in IV-PCA, which has been shown to be effective for pain control with fewer side effects after the Nuss procedure in pediatric patients.3
Ketamine has analgesic properties in subanesthetic doses.5,7 Small doses of ketamine act specifically to prevent opioid tolerance and pain sensitization.7 Low-dose ketamine is defined as a single injection of less than 1 mg/kg or continuous infusion of less than 20 µg/kg/min.12 The incidence of psychomimetic effects and cognitive impairment is negligible at doses less than 2.5 µg/kg/min and increases with higher doses of ketamine.12 It has been reported that a 2.5 µg/kg/min infusion of ketamine on IV-PCA can decrease morphine consumption and the incidence of nausea.13 Therefore, we decided to test a basal infusion rate of ketamine at 2.5 µg/kg/min (=0.15 mg/kg/hr).
In review, Subramaniam, et al.14 concluded that a small dose of ketamine is a safe and useful adjuvant to standard IV or epidural opioid-analgesia, however, adding ketamine to IV-PCA with morphine showed no beneficial effect in postoperative pain control. Subsequently, conflicting results exist concerning the potential benefits of adding ketamine to opioids in IV-PCA for postoperative pain control.8-20 The results can be categorized by several types of surgery.
For thoracic surgery, Nesher, et al.8,15 studied patients undergoing thoracotomy and reported that a bolus of 5 mg of ketamine plus 1 mg of morphine IV-PCA (lockout 7 min) resulted in a reduction in pain score and a 45-50% reduction in morphine consumption with cardiovascular stability and better respiratory parameters. Michelet, et al.9 studied patients undergoing thoracic surgery and reported that adding ketamine 1 mg/mL to morphine 1 mg/mL IV-PCA (a bolus of 0.015 mL/kg per every 10 min) resulted in reductions in morphine consumption and pain score and a reduction in nocturnal desaturation.
The benefit of adding ketamine to morphine in IV-PCA for orthopedic surgery remains unclear. Javery, et al.16 studied patients undergoing lumbar microdiscectomy and reported that ketamine 1 mg/mL added to morphine 1 mg/mL IV-PCA (bolus 1 mL, lockout 6 min) reduced pain scores and halved morphine consumption. Kollender, et al.17 studied patients undergoing orthopedic-oncological surgery and found that a bolus of 5 mg ketamine plus 1 mg morphine IV-PCA (lockout 7 min) resulted in a 60% reduction in morphine consumption and reductions in pain score and additional analgesic use. However, Sveticic, et al.10 studied patients undergoing major orthopedic surgery and reported that a bolus of 1.5 mg ketamine plus 1.5 mg morphine in IV-PCA (lockout 8 min) failed to demonstrate beneficial effects in morphine consumption and pain control.
The benefit of adding ketamine to morphine in IV-PCA for abdominal surgery also remains unclear. Adriaenssens, et al.13 studied patients undergoing abdominal surgery and reported that continuous infusion of ketamine 2.5 µg/kg/min with morphine IV-PVA (loading dose 3 mg, bolus 1 mg, lockout 8 min) reduced pain score and morphine consumption. Unlügenç, et al.18 studied patients after major abdominal surgery and found that ketamine 1 mg/mL added to tramadol 5 mg/mL IV-PCA (loading dose 1 mg/kg, bolus 0.2 mg/kg, lockout 20 min, background infusion 0.4 mg/kg/h) improved analgesia and reduced tramadol consumption. However, investigating patients undergoing total abdominal hysterectomy, Murdoch, et al.19 did not find any benefit of adding ketamine 0.75 mg/mL to morphine 1 mg/mL IV-PCA (10 mL/m2 body surface area of loading dose, bolus 1 mL, lockout 5 min, background infusion 1 mL/h). Reeves, et al.20 found no pain-reducing or morphine-sparing benefits of ketamine bolus 1 mg/mL plus morphine 1 mg/mL IV-PCA (bolus, lockout, and background infusions were determined by the anesthesiologists) in patients after major abdominal surgery.
In our study, adding ketamine to fentanyl IV-PCA reduced the cumulative fentanyl consumption by approximately 22%, reduced pain scores, and reduced additional analgesic use, with parent satisfaction in pain control. Although these reduction levels were different, the results appear to be in agreement with prior studies.8,9,13,15-18 We conclude that there is a benefit to adding ketamine to opioids for IV-PCA for thoracic surgery.8,9,15 However, controversies remain regarding the benefits of adding ketamine to opioids for IV-PCA for abdominal and orthopedic surgery.10-14,16-20 These contradictory results may reflect differences in the methodology of each study, degree of postoperative pain, nature of surgical procedure, and nature and source of postoperative pain.
There is a significant incidence of nausea/vomiting when fentanyl IV-PCA is used postoperatively after the Nuss procedure, observed in 30-50% pediatric patients.2-4 In our study, the incidence of nausea/vomiting was 53% in the fentanyl group and 23% in the fentanyl plus ketamine group. Our results agree with prior studies that adding ketamine to morphine IV-PCA reduces the incidence of nausea/vomiting.13,15-17 However, nausea/vomiting can be induced by ketamine itself in a dose dependent pattern. Badrinath, et al.21 investigated the dose-dependent effects of ketamine during propofol sedation and reported that 24 µg/kg/min of ketamine, could induce nausea/vomiting. In our study, 2.5 µg/kg/min of ketamine, was low enough to avoid emetic effects. The incidence of patients who used the antiemetic, ondansetron, during the 48 hours after surgery was 10% in the fentanyl plus ketamine group and was lower than 37% in the fentanyl group.
Pruritus is a common side effect of opioid administration, however, the incidence and severity is lower in the IV route than in the epidural or intrathecal route.22 The incidence of pruritus is 0-10% when fentanyl IV-PCA is used after the Nuss procedure.2,3 In our study, the incidence of pruritus was 13% in the fentanyl group and 7% in the fentanyl plus ketamine group. Although, the incidence was almost two times higher in the fentanyl group than in the fentanyl plus ketamine group, the incidences of both groups were too low to differentiate with statistical significance in either group. There were no instances of severe pruritus which required treatment in both groups.
Thoracic surgery can lead to respiratory depression due to severe pain, inhibiting thoracic expansion while breathing. Adequate analgesia to reduce postoperative pain enhances the ability to breathe deeply and cough effectively, alleviating respiratory depression.23 However, opioids in large doses can worsen respiration rates, oxygenation, and ventilation. Therefore, a reasonable amount of opioids and adjuvant to opioids are important to pain control as well as respiration. High doses of ketamine can have respiratory depressive effects, but respiratory depression associated with small doses of ketamine is minimal.8,9,15,21 Ketamine also sustains the patency of small and large airways, thus preventing obstruction, including the kind that can occur in patients under the effect of opioids. In our study, the infusion rates of 0.5 µg/kg/hr of fentanyl and 0.15 mg/kg/hr of ketamine were low enough to avoid respiratory depression. These features lead to better respiration and a probable preservation of more than 90% of pulse oximetry saturation during the postoperative period.
Ketamine is associated with a risk of psychomimetic effects, including hallucination, vivid dreaming, sedation, and dizziness. However, the safety of adding low-dose ketamine to morphine for postoperative IV-PCA regarding its psychomimetic effects is well established.8,13,15,17,18 Badrinath, et al.21 investigated the dose-dependent effects of ketamine and reported that a dose of 24 µg/kg/min can increase psychomimetic effects. Edwards, et al.24 used a dose-finding approach to study the combination of morphine and ketamine in IV-PCA for postoperative pain in elderly patients and found that vivid dreaming was a problem at doses of ketamine of 7.8 µg/kg/min. The incidence of psychomimetic symptoms associated with ketamine is lower in children than in adults.25 There were no hallucinations or cases of dreaming in our experiment. The dose of ketamine of 0.15 mg/kg/hr (=2.5 µg/kg/min) was low enough to avoid psychomimetic effects.
To the best of our knowledge, this study is the first report on the effects of adding ketamine to fentanyl IV-PCA for postoperative pain control after the Nuss procedure in children. We observed good parent satisfaction with pain control, reduced pain scores, reduced need for additional analgesic, lower fentanyl consumption, reduced incidence of nausea/vomiting, and reduced need for antiemetics, without increased side effects. Therefore, if fentanyl IV-PCA is considered for postoperative pain control following the Nuss procedure in children, we recommend adding low-dose ketamine to the fentanyl IV-PCA.
Figures and Tables
 | Fig. 1Flow diagram of the progress through the trial. Group F, fentanyl group; Group FK, fentanyl plus ketamine group. |
References
1. Nuss D, Kelly RE Jr, Croitoru DP, Katz ME. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998. 33:545–552.

2. Butkovic D, Kralik S, Matolic M, Kralik M, Toljan S, Radesic L. Postoperative analgesia with intravenous fentanyl PCA vs epidural block after thoracoscopic pectus excavatum repair in children. Br J Anaesth. 2007. 98:677–681.

3. Ahn KR, Chung JW, Kwon JH, Kang KS, Lee JS, Yoo SH, et al. Intravenous patient-controlled analgesia using fentanyl after nuss procedure in pediatric patients undergoing pectus excavatum repair. Korean J Anesthesiol. 2005. 49:624–629.

4. Rugyte DC, Kilda A, Karbonskiene A, Barauskas V. Systemic postoperative pain management following minimally invasive pectus excavatum repair in children and adolescents: a retrospective comparison of intravenous patient-controlled analgesia and continuous infusion with morphine. Pediatr Surg Int. 2010. 26:665–669.

5. Visser E, Schug SA. The role of ketamine in pain management. Biomed Pharmacother. 2006. 60:341–348.
6. Phillips WJ, Currier BL. Analgesic pharmacology: II. Specific analgesics. J Am Acad Orthop Surg. 2004. 12:221–233.

7. Laulin JP, Maurette P, Corcuff JB, Rivat C, Chauvin M, Simonnet G. The role of ketamine in preventing fentanyl-induced hyperalgesia and subsequent acute morphine tolerance. Anesth Analg. 2002. 94:1263–1269.

8. Nesher N, Ekstein MP, Paz Y, Marouani N, Chazan S, Weinbroum AA. Morphine with adjuvant ketamine vs higher dose of morphine alone for immediate postthoracotomy analgesia. Chest. 2009. 136:245–252.

9. Michelet P, Guervilly C, Hélaine A, Avaro JP, Blayac D, Gaillat F, et al. Adding ketamine to morphine for patient-controlled analgesia after thoracic surgery: influence on morphine consumption, respiratory function, and nocturnal desaturation. Br J Anaesth. 2007. 99:396–403.

10. Sveticic G, Farzanegan F, Zmoos P, Zmoos S, Eichenberger U, Curatolo M. Is the combination of morphine with ketamine better than morphine alone for postoperative intravenous patient-controlled analgesia? Anesth Analg. 2008. 106:287–293.

11. Jensen LL, Handberg G, Helbo-Hansen HS, Skaarup I, Lohse T, Munk T, et al. No morphine sparing effect of ketamine added to morphine for patient-controlled intravenous analgesia after uterine artery embolization. Acta Anaesthesiol Scand. 2008. 52:479–486.

12. Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: a review of current techniques and outcomes. Pain. 1999. 82:111–125.

13. Adriaenssens G, Vermeyen KM, Hoffmann VL, Mertens E, Adriaensen HF. Postoperative analgesia with i.v. patient-controlled morphine: effect of adding ketamine. Br J Anaesth. 1999. 83:393–396.

14. Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review. Anesth Analg. 2004. 99:482–495.

15. Nesher N, Serovian I, Marouani N, Chazan S, Weinbroum AA. Ketamine spares morphine consumption after transthoracic lung and heart surgery without adverse hemodynamic effects. Pharmacol Res. 2008. 58:38–44.

16. Javery KB, Ussery TW, Steger HG, Colclough GW. Comparison of morphine and morphine with ketamine for postoperative analgesia. Can J Anaesth. 1996. 43:212–215.

17. Kollender Y, Bickels J, Stocki D, Maruoani N, Chazan S, Nirkin A, et al. Subanaesthetic ketamine spares postoperative morphine and controls pain better than standard morphine does alone in orthopaedic-oncological patients. Eur J Cancer. 2008. 44:954–962.

18. Unlügenç H, Gündüz M, Ozalevli M, Akman H. A comparative study on the analgesic effect of tramadol, tramadol plus magnesium, and tramadol plus ketamine for postoperative pain management after major abdominal surgery. Acta Anaesthesiol Scand. 2002. 46:1025–1030.

19. Murdoch CJ, Crooks BA, Miller CD. Effect of the addition of ketamine to morphine in patient-controlled analgesia. Anaesthesia. 2002. 57:484–488.

20. Reeves M, Lindholm DE, Myles PS, Fletcher H, Hunt JO. Adding ketamine to morphine for patient-controlled analgesia after major abdominal surgery: a double-blinded, randomized controlled trial. Anesth Analg. 2001. 93:116–120.

21. Badrinath S, Avramov MN, Shadrick M, Witt TR, Ivankovich AD. The use of a ketamine-propofol combination during monitored anesthesia care. Anesth Analg. 2000. 90:858–862.

22. Werawatganon T, Charuluxanun S. Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra-abdominal surgery. Cochrane Database Syst Rev. 2005. CD004088.

23. Fléron MH, Weiskopf RB, Bertrand M, Mouren S, Eyraud D, Godet G, et al. A comparison of intrathecal opioid and intravenous analgesia for the incidence of cardiovascular, respiratory, and renal complications after abdominal aortic surgery. Anesth Analg. 2003. 97:2–12.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download