Abstract
Purpose
Spontaneous bacterial peritonitis (SBP) frequently develops in patients with liver cirrhosis; however, there is little data to suggest whether the acquisition site of infection influences the prognosis. This study compared the bacteriology, clinical characteristics and treatment outcomes of community-acquired SBP (CA-SBP) and nosocomial SBP (N-SBP).
Materials and Methods
The medical records of 130 patients with hepatitis B virus (HBV)-related liver cirrhosis, who had experienced a first episode of SBP between January 1999 and December 2008, were reviewed.
Results
The study population included 111 (85.4%) patients with CA-SBP and 19 (14.6%) patients with N-SBP. Baseline and microbiological characteristics as well as clinical course, including in-hospital mortality, did not differ between patients with CA-SBP and those with N-SBP (all p>0.05). The median survival time was 6.5 months, and 117 (90.0%) patients died during the follow-up period. Patients with CA-SBP and N-SBP survived for median periods of 6.6 and 6.2 months, respectively, without significant difference (p=0.569). Time to recurrence did not differ between patients with CA-SBP and N-SBP (4.7 vs. 3.6 months, p=0.925).
Conclusion
The acquisition site of infection did not affect clinical outcomes for patients with HBV-related liver cirrhosis who had experienced their first episode of SBP. Third-generation cephalosporins may be effective in empirically treating these patients, regardless of the acquisition site of the infection.
Spontaneous bacterial peritonitis (SBP) is an ascitic fluid infection without a definitive, surgically treatable, intra-abdominal source and is a drastic complication of end-stage liver disease, occurring in 10 to 25% of cirrhotic patients with ascites.1,2 Although mortality related to SBP has markedly decreased over the last 3 decades, due to earlier recognition of the infection followed by administration of effective antibiotics, it continues to be high, ranging from 20 to 40%.3-5 In addition, the one-year survival rate after recovery from the first episode of SBP is only 30 to 40%.6
Intestinal bacterial overgrowth and subsequent translocation of bacteria from the intestines to the mesenteric lymph nodes is known to be a critical step in the pathogenesis of SBP.7,8 In patients with liver cirrhosis, impairment of the immune system, due to complement deficiencies and neutrophilic malfunction, hampers clearing bacteria from the ascites, facilitating the development of SBP.9,10 Thus, patients with liver cirrhosis are susceptible to bacterial infections both inside and outside the hospital.
To date, few studies have investigated the effects of the acquisition site of infection (community-acquired vs. nosocomial) on clinical outcomes in patients with liver cirrhosis with accompanying SBP.11-13 However, factors such as various etiologies of liver cirrhosis, history of previous SBP,11,13 and hepatocellular carcinoma (HCC) at the time of SBP diagnosis might have confounded exact comparisons between patients with community-acquired SBP (CA-SBP) and nosocomial SBP (N-SBP) in previous studies.11-13 Furthermore, differences in baseline liver function at the time of CA-SBP or N-SBP diagnosis11 might have disturbed exact comparisons of their prognoses.
Therefore, this study focused on patients with hepatitis B virus (HBV)-related liver cirrhosis who had experienced their first episode of SBP. We compared microbiological and clinical characteristics as well as treatment outcomes (in-hospital clinical course, time to recurrence, and overall survival) of patients with CA-SBP and N-SBP.
The medical records of 130 patients with HBV-related liver cirrhosis who had experienced their first episode of SBP and were treated at either Severance Hospital (College of Medicine, Yonsei University, Seoul, Korea) or at the National Health Insurance Corporation Ilsan Hospital (Goyang, Korea) between January 1999 and December 2008, were reviewed. Patients with a history of previous SBP or non-HBV etiologies of cirrhosis, such as hepatitis C virus or alcohol abuse, were excluded. Patients with coexisting HCC at the time of SBP diagnosis and those who underwent a liver transplantation during the follow-up period were also excluded to remove confounding effects of these factors on survival. In addition, patients whose ascites were caused by tuberculosis or malignancy or whose culture results suggested secondary bacterial peritonitis (polymicrobial infection) or contamination by skin or medical appliances (coagulase-negative staphylococci, corynebacterium, propionibacterium, or bacillus species) were excluded. The protocol of this study was consistent with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the independent institutional review boards of each institute.
The diagnostic criteria for SBP were a positive ascitic fluid culture with an elevated (>250 cells/mm3) ascitic polymorphonuclear leukocyte (PMN) count and/or a positive ascitic fluid culture.14-16 SBP diagnosis within the first 48 hours of hospitalization was categorized as CA-SBP, while diagnosis more than 48 hours after hospitalization was defined as N-SBP.13,17
Variceal bleeding, hepatic encephalopathy (HE), renal failure, blood culture positivity, antibiotic switching during hospitalization and in-hospital mortality were reviewed to compare the clinical courses of patients with CA-SBP and N-SBP. An esophago-gastro-duodenoscopy was required to confirm variceal bleeding. HE was defined as an episode of asterixis, mental confusion, loss of orientation, excitation or abnormal behavior.18 Renal failure was defined as >50% increase (vs. pretreatment value) of blood urea nitrogen or serum creatinine level of more than 30 mg/dL or 1.5 mg/dL, respectively, in patients with normal renal function at the time of enrollment. For patients with preexisting renal impairment, a diagnosis of renal failure required an increase in blood urea nitrogen or serum creatinine level greater than 50% from baseline.19
Bacterial identification and antibiotic susceptibility tests were performed according to standard procedures established by the Clinical and Laboratory Standards Institute, following our previously described methods.20
Paracentesis was carried out using a 23-gauge sterile needle under local anesthesia with lidocaine. After withdrawal from the abdomen, this skin needle was replaced with a sterile needle to minimize contamination by skin flora. Within 3 hours of aspiration, the obtained peritoneal fluids were sent to the laboratory to calculate the PMN counts and to perform gram staining. Ascitic fluid samples (10 mL) were then inoculated into aerobic and anaerobic blood bottles (bioMerieux, Durham, NC, USA) and cultured with an automated BacT/Alert 3D culture system (bioMerieux, Durham, NC, USA). Conventional culture methods (i.e., inoculating blood agar, MacConkey agar, and phenylethanol agar and thioglycollate broth) were used on the remaining fluid from each sample. The conventional agar and broth media were incubated at 35℃ for up to 3 days before being discarded as negative.
According to the guidelines of our institute, all patients with ascites upon admission routinely underwent paracentesis within 24 h of admission.21 If the symptoms and laboratory results were indicative of SBP, 2 g cefotaxime every 8 hours was administered as the initial antibiotic treatment for all patients and was continued until recovery, antibiotic switching, or death. Follow-up paracentesis was scheduled 48 hours after antibiotic administration to evaluate treatment response or when clinical or laboratory findings did not show typical improvement. Treatment failure was defined as a decrease of less than 50% in ascitic fluid PMN count, in cases where follow-up paracentesis was performed.21 Antibiotics were switched according to the culture and sensitivity results of the initial ascitic fluid test, treatment failure, or persistent clinical deterioration. Intravenous albumin was infused using the recommended protocol.19 Recovery from SBP was clinically assessed by the disappearance of symptoms or by negative cultures and reduction in ascitic fluid PMN count to less than 250/mm3.22 Norfloxacin was administered after recovery for prophylaxis.23
Survival time was calculated from the date of the first SBP diagnosis to death. In-hospital mortality was assessed by counting deaths during hospitalization, and overall mortality was evaluated by counting the number of deaths that occurred throughout the entire follow-up period (to December 2009). Time to recurrence was defined as the period between discharge from the hospital after the first SBP episode to the next SBP episode.
All variables are reported as mean±standard deviation, median (range), or number (%). Independent t-tests were used to compare continuous variables, and chi-square tests were used for categorical variables. Binary logistic regression analysis was used to identify independent predictors for in-hospital mortality. Independent prognostic factors for overall survival were identified with a proportional hazards Cox regression model, and corresponding hazard ratios (HR) and 95% confidence intervals (CI) were calculated. The cumulative probability of death or disease recurrence was analyzed by the Kaplan-Meier method. Time to recurrence and overall survival were compared between patients with CA-SBP and N-SBP using the log rank test. All statistical analyses were performed using the SPSS software package (Version 12.0, SPSS Inc., Chicago, IL, USA), and two-sided p-values <0.05 were considered statistically significant.
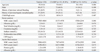
Table 1 shows the baseline characteristics of the study population at the time of diagnosis. A total of 130 patients (88 male and 42 female) had a mean age of 53.3 years (range, 44.9-61.7 years). One hundred and eleven (85.4%) patients had CA-SBP, whereas 19 (14.6%) had N-SBP. Patients with N-SBP were initially admitted for the management of jaundice (n=7), gastrointestinal bleeding (n=6), poor oral intake (n=3), and HE (n=3). Among six patients with N-SBP who were admitted due to gastrointestinal bleeding, two were given ciprofloxacin on admission as a prophylactic. However, the antibiotic agent was changed to cefotaxime after they were diagnosed with N-SBP. No significant differences were found between patients with CA-SBP and N-SBP in regards to age, gender, history of previous variceal bleeding or HE, and Child-Pugh scores (all p>0.05). Although the results of the serological and ascitic fluid tests were comparable, the average serum white blood cell count was significantly higher in patients with CA-SBP than those with N-SBP (8197±4978 vs. 4780±2244/mm3, p=0.006), while the mean serum sodium level was significantly higher in patients with N-SBP than those with CA-SBP (135.4±5.8 vs. 131.6±5.8 mmol/L, p=0.007).
All 111 patients with CA-SBP underwent their first paracentesis within 24 hours of hospital admission [median, 2 h (range, 1-20)] and were diagnosed with SBP. By contrast, all 19 N-SBP patients with accompanying ascites showed negative results for SBP on the initial admission paracentesis [median, 2 h (range, 1-24)]. However, due to fever (n=8), abdominal distension (n=7), and abdominal pain (n=4), N-SBP patients received a second paracentesis procedure. After 8.2 days (range, 3.8-12.2) of hospital admission, all 19 patients were diagnosed with N-SBP.
A scheduled follow-up paracentesis at 48 hours after the initial antibiotic administration was performed in 76 patients [67 (60.4%) with CA-SBP vs. 9 (47.4%) with N-SBP, p=0.321]. In another 6 (4.6%) patients, paracentesis was repeated because they did not show clinical improvement in spite of a decrease in ascitic fluid PMN count [4 (3.6%) with CA-SBP vs. 2 (10.5%) with N-SBP, p=0.092].
The organisms cultured from the ascitic fluid are listed in Table 2. Pathogens were isolated in 37 (28.5%) of 130 ascitic fluid samples [32 (28.8%) with CA-SBP vs. 5 (26.3%) with N-SBP]. The most common organism in patients with CA-SBP and N-SBP was Escherichia coli [20 (62.5%) with CA-SBP vs. 3 (60.0%) with N-SBP]. No significant differences in microorganisms were identified between the two groups (p=0.961).
Table 3 shows the clinical courses of patients with CA-SBP and N-SBP during hospitalization. The incidence of liver-related complications such as variceal bleeding, HE, and renal failure did not differ between the two groups (all p>0.05). Furthermore, blood culture positivity (p=0.578), antibiotic switching (p=0.066), and in-hospital mortality (p=0.163) did not differ.
Antibiotics were switched in 11 (8.5%) patients, due to cefotaxime resistance, treatment failure, or persistent clinical deterioration. Cefotaxime resistance developed in three (8.1%) of 37 patients with positive ascitic fluid culture [2 (6.3%) with CA-SBP vs. 1 (20.0%) with N-SBP, p=0.233]. Cefotaxime was switched to carbapenem in 10 patients and to ciprofloxacin in one. Among these 11 patients, nine patients died during hospitalization, while two recovered.
Table 4 presents the results of the univariate and multivariate analyses to identify the independent predictors of in-hospital mortality. The univariate analysis demonstrated that variceal bleeding during hospitalization and ascitic fluid culture positivity significantly predicted in-hospital mortality (p=0.035 and p=0.031, respectively). However, multivariate analysis identified ascitic fluid culture positivity as the only independent predictor of in-hospital mortality (p=0.036; HR, 5.392; 95% CI, 1.208-24.061).
In total, 104 (93.7%) patients with CA-SBP and 16 (84.2%) with N-SBP survived their first episode of SBP. After discharge, SBP recurred in 42 (40.4%) of 104 patients with CA-SBP and 6 (37.5%) of 16 with N-SBP after a median period of 4.7 months (range, 0.8-29.5) and 3.6 months (range, 1.3-12.8), respectively (p=0.925) (Fig. 1).
The median survival time of the study population was 6.5 months (range, 0.1-136.1); 117 (90.0%) patients had died by the end of the follow-up period. The median survival time of patients with CA-SBP was 6.6 months (range, 0.1-128.1), and that of patients with N-SBP was 6.2 months (range, 0.2-136.1) (p=0.569) (Fig. 2). The 1-, 6-, and 12-month cumulative mortality rates of patients with CA-SBP were 7.2%, 48.7% and 64.2%, respectively, and those for patients with N-SBP were 15.8%, 47.6%, and 70.9%, respectively. The Cox-regression hazard model revealed that Child-Pugh score (p=0.001; HR, 1.312; 95% CI, 1.122-1.536) and serum sodium level (p=0.007; HR, 0.946; 95% CI, 0.909-0.985) were independent predictors of overall survival (Table 5).
Causes of in-hospital and overall mortality are summarized in Table 6. Septic shock was the most common cause of in-hospital mortality [three (42.9%) with CA-SBP and one (33.3%) with N-SBP, p=0.087]. Concerning overall mortality, gastrointestinal bleeding (21.5%), hepatic failure (21.5%) and septic shock (20.4%) were common in patients with CA-SBP, whereas gastrointestinal bleeding (21.4%), septic shock (21.4%), renal failure (21.4%), and HE (21.4%) were common in those with N-SBP. Causes of in-hospital and overall mortality did not significantly differ between the two groups (p=0.917 and 0.375, respectively).
Patients with CA-SBP and N-SBP in this study showed similar clinical and microbiological characteristics, clinical course during hospitalization, time to recurrence and overall survival, as well as causes of mortality. These results suggest that these two types of SBP are not different disease entities and, furthermore, that the acquisition site of the pathogen (community-acquired vs. nosocomial) does not affect the prognosis of SBP patients.
To date, few studies have compared the characteristics of CA-SBP and N-SBP. Bert, et al.13 found that nosocomial isolates were significantly more resistant to amoxicillin/clavulanic acid and cefotaxime than community-acquired isolates and, thus, insisted that N-SBP requires a wider spectrum of antibiotics than CA-SBP. Moreover, Cheong, et al.11 concluded that nosocomial acquisition of SBP pathogens adversely affected the clinical outcomes of SBP, and that N-SBP mortality was accordingly higher. In contrast, Song, et al.12 reported that acquisition sites of infection did not have prognostic significance in SBP, and Umgelter, et al.17 concluded that the microbiological patterns and outcomes of CA-SBP and N-SBP did not differ. Therefore, differences in the prognosis of CA-SBP and N-SBP still remain unresolved. We believe that the discrepancies among these previous studies are a result of heterogeneity in their study populations caused by differences in baseline liver functions between patients with CA-SBP and N-SBP, the inclusion of patients with a history of previous SBP, coexisting HCC in some patients at baseline, and various etiologies of liver cirrhosis.11-13
Our study differs from previous studies in several ways. First, the baseline characteristics, including liver function, were similar between enrolled patients with CA-SBP and N-SBP. Therefore, we could exclude the potential confounding effects of different liver function on the prognosis of CA-SBP and N-SBP. Although a previous study revealed that patients with N-SBP showed poorer prognosis and were infected by more virulent organisms than those with CA-SBP,11 baseline liver function was more favorable in patients with CA-SBP. Thus, the poor prognosis for patients with N-SBP might have been caused by poorer baseline liver function alone, and not by any inherent characteristics of N-SBP. According to our results, discrimination between CA-SBP and N-SBP may be meaningless, at least for the first episode of SBP, when liver function is relatively well preserved, indicating that CA-SBP and N-SBP may be of the same spectrum of SBP and that vulnerability to SBP is determined only by liver function status throughout the course of liver disease. This idea is also supported by the fact that SBP is a problem of increased gut permeability and bacterial translocation resulting from the intrinsic pathophysiological process of each individual patient and is not caused by pathogens from outside of the body.24
Second, our study included only patients who had experienced their first episode of SBP. The chance of exposure to more resistant pathogen strains increases as patients experience repeated SBP events and admissions.17 Therefore, it is difficult to purely compare the effect of acquisition site of infection on prognosis, if a study population includes patients with a history of previous SBP. However, certain environmental changes occurring after the first episode of SBP, such as changes in intestinal bacteria leading to vulnerability to invasive pathogens, a weakened immune system by progressive deterioration of liver function, and interactions between these factors, might result in the differing prognoses between CA-SBP and N-SBP. Thus, further investigations of microorganism alterations and antibiotic susceptibility in recurring episodes of SBP after surviving the first episode of SBP should be performed.
Third, this study excluded patients with coexisting HCC at the time of SBP diagnosis, which might influence the natural course of SBP, because increased hospitalization periods for HCC management can increase the chance of developing N-SBP.25 Finally, we focused on patients with HBV-related cirrhosis because the natural history of patients with cirrhosis differs according to the etiology of the liver disease,18 and HBV is the most prevalent (57-73%) etiology for liver cirrhosis in Korea.26,27 Hepatitis C virus-related cirrhosis usually progresses more slowly than HBV-related cirrhosis, and abstinence from alcohol intake can prolong the survival of patients with alcohol-related cirrhosis.18,28
Although some studies have found that Streptococcus pneumoniae is the most common organisms in patients with CA-SBP and that higher levels of gram-positive pathogens are present in those with N-SBP,29 the most commonly occurring organisms in SBP are usually enteric gram-negative rods such as Escherichia coli.30 Our study also identified gram-negative pathogens as the dominant pathogens in both patients with CA-SBP and patients with N-SBP. Also, no significant differences in the isolation of microorganisms were found between two groups. This result, which again demonstrates the similarities of CA-SBP and N-SBP, may be explained by the blurring of environmental boundaries between CA-SBP and N-SBP, as patients with cirrhosis accompanied by ascites receive frequent hospital assistance, including outpatient visits, home nursing care by health-care providers, and repeated hospital admission.31
Since 1985, third-generation cephalosporins have been the most frequently used antibiotics for the management of SBP.32 All patients in the present study initially received cefotaxime. Treatment failure and cefotaxime resistance occurred in 3.9% and 8.1% of patients respectively, and the antibiotic regimen was switched in only 8.5% of patients. These results indicate that our study population was not yet susceptible to third-generation cephalosporin-resistant bacterial infection, and that the third-generation cephalosporin cefotaxime is sufficient as a first-line treatment in patients experiencing their first episode of SBP, regardless of the acquisition site of infection.
The acquisition site of SBP pathogen did not affect the clinical course during hospitalization or in-hospital mortality in our study. Ascitic fluid culture positivity was the only significant predictor of in-hospital mortality.20 Furthermore, only Child-Pugh scores and serum sodium levels significantly predicted overall survival, while the acquisition site of the infection did not. Moreover, we found no statistical differences between patients with CA-SBP and N-SBP regarding overall survival, time to recurrence, and causes of mortality. All these results suggest that these two types of SBP are similar disease entities.
Despite the unique features of the present study, there are some limitations. First, because this study was retrospectively designed, the sample size is relatively small, particularly the group of patients with N-SBP. Moreover, follow-up paracentesis, 48 hours after antibiotics administration, was performed in only 58.5% of patients (60.4% with CA-SBP and 47.4% with N-SBP). Lastly, the microbiology of SBP may have changed over the long study period from 1999 to 2008. We were not able to stratify the organisms of SBP according to the time period because the number of isolated organisms was too small. Therefore, future prospectively designed studies incorporating a larger number of patients should be performed to overcome these limitations.
In conclusion, the acquisition site of infection (community-acquired vs. nosocomial) did not affect the clinical outcomes and prognosis of patients with HBV-related liver cirrhosis who had experienced their first episode of SBP. Third-generation cephalosporins may be effective in empirically treating these patients, regardless of the acquisition site of infection.
Figures and Tables
 | Fig. 1Cumulative recurrence-free curves of patients with CA-SBP and patients with N-SBP. Time to recurrence was not different between the groups [median 4.7 months (range, 0.8-29.5) vs. 3.6 months (range, 1.3-12.8), p=0.925]. CA-SBP, community acquired spontaneous bacterial peritonitis; N-SBP, nosocomial spontaneous bacterial peritonitis; |
 | Fig. 2Cumulative overall survival curves of patients with CA-SBP and patients with N-SBP. Overall survival was not different between the groups [median 6.6 months (range, 0.1-128.1) vs. 6.2 months (range, 0.2-136.1), p=0.569]. CA-SBP, community acquired spontaneous bacterial peritonitis; N-SBP, nosocomial spontaneous bacterial peritonitis; |
Table 1
Baseline Characteristics of Patients with CA-SBP and Patients with N-SBP at the Time of Diagnosis
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare technology R & D project, Ministry of Health and Welfare, Republic of Korea (A102065).
References
1. Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology. 1990. 98:127–133.

2. Runyon BA. Spontaneous bacterial peritonitis: an explosion of information. Hepatology. 1988. 8:171–175.

4. Navasa M, Follo A, Llovet JM, Clemente G, Vargas V, Rimola A, et al. Randomized, comparative study of oral ofloxacin versus intravenous cefotaxime in spontaneous bacterial peritonitis. Gastroenterology. 1996. 111:1011–1017.

5. Runyon BA, McHutchison JG, Antillon MR, Akriviadis EA, Montano AA. Short-course versus long-course antibiotic treatment of spontaneous bacterial peritonitis. A randomized controlled study of 100 patients. Gastroenterology. 1991. 100:1737–1742.

6. Altman C, Grangé JD, Amiot X, Pelletier G, Lacaine F, Bodin F, et al. Survival after a first episode of spontaneous bacterial peritonitis. Prognosis of potential candidates for orthotopic liver transplantation. J Gastroenterol Hepatol. 1995. 10:47–50.

8. Guarner C, Runyon BA, Young S, Heck M, Sheikh MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol. 1997. 26:1372–1378.

9. Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis. 2000. 182:526–533.

10. Runyon BA. Patients with deficient ascitic fluid opsonic activity are predisposed to spontaneous bacterial peritonitis. Hepatology. 1988. 8:632–635.

11. Cheong HS, Kang CI, Lee JA, Moon SY, Joung MK, Chung DR, et al. Clinical significance and outcome of nosocomial acquisition of spontaneous bacterial peritonitis in patients with liver cirrhosis. Clin Infect Dis. 2009. 48:1230–1236.

12. Song JY, Jung SJ, Park CW, Sohn JW, Kim WJ, Kim MJ, et al. Prognostic significance of infection acquisition sites in spontaneous bacterial peritonitis: nosocomial versus community acquired. J Korean Med Sci. 2006. 21:666–671.

13. Bert F, Andreu M, Durand F, Degos F, Galdbart JO, Moreau R, et al. Nosocomial and community-acquired spontaneous bacterial peritonitis: comparative microbiology and therapeutic implications. Eur J Clin Microbiol Infect Dis. 2003. 22:10–15.

14. Terg R, Levi D, Lopez P, Rafaelli C, Rojter S, Abecasis R, et al. Analysis of clinical course and prognosis of culture-positive spontaneous bacterial peritonitis and neutrocytic ascites. Evidence of the same disease. Dig Dis Sci. 1992. 37:1499–1504.

15. Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montgomerie JZ. Spontaneous bacterial peritonitis. Hepatology. 1982. 2:399–407.

16. Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology. 1984. 4:1209–1211.

17. Umgelter A, Reindl W, Miedaner M, Schmid RM, Huber W. Failure of current antibiotic first-line regimens and mortality in hospitalized patients with spontaneous bacterial peritonitis. Infection. 2009. 37:2–8.

18. Kim SU, Han KH, Nam CM, Park JY, Kim do Y, Chon CY, et al. Natural history of hepatitis B virus-related cirrhotic patients hospitalized to control ascites. J Gastroenterol Hepatol. 2008. 23:1722–1727.

19. Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999. 341:403–409.

20. Kim SU, Kim do Y, Lee CK, Park JY, Kim SH, Kim HM, et al. Ascitic fluid infection in patients with hepatitis B virus-related liver cirrhosis: culture-negative neutrocytic ascites versus spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 2010. 25:122–128.

21. Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000. 32:142–153.

22. al Amri SM, Allam AR, al Mofleh IA. Spontaneous bacterial peritonitis and culture negative neutrocytic ascites in patients with non-alcoholic liver cirrhosis. J Gastroenterol Hepatol. 1994. 9:433–436.

23. Novella M, Solà R, Soriano G, Andreu M, Gana J, Ortiz J, et al. Continuous versus inpatient prophylaxis of the first episode of spontaneous bacterial peritonitis with norfloxacin. Hepatology. 1997. 25:532–536.

24. Llovet JM, Bartolí R, March F, Planas R, Viñado B, Cabré E, et al. Translocated intestinal bacteria cause spontaneous bacterial peritonitis in cirrhotic rats: molecular epidemiologic evidence. J Hepatol. 1998. 28:307–313.

25. Park YH, Lee HC, Song HG, Jung S, Ryu SH, Shin JW, et al. Recent increase in antibiotic-resistant microorganisms in patients with spontaneous bacterial peritonitis adversely affects the clinical outcome in Korea. J Gastroenterol Hepatol. 2003. 18:927–933.

26. Kim YS, Um SH, Ryu HS, Lee JB, Lee JW, Park DK, et al. The prognosis of liver cirrhosis in recent years in Korea. J Korean Med Sci. 2003. 18:833–841.

27. Han YS, Kim BH, Baek IY, Lee DK, Kim KJ, Dong SH, et al. The change of the etiology, complications and cause of death of the liver cirrhosis in 1990s. Korean J Hepatol. 2000. 6:328–339.
28. Kaymakoglu S, Eraksoy H, Okten A, Demir K, Calangu S, Cakaloglu Y, et al. Spontaneous ascitic infection in different cirrhotic groups: prevalence, risk factors and the efficacy of cefotaxime therapy. Eur J Gastroenterol Hepatol. 1997. 9:71–76.

29. Johnson CC, Baldessarre J, Levison ME. Peritonitis: update on pathophysiology, clinical manifestations, and management. Clin Infect Dis. 1997. 24:1035–1045.

30. Bhuva M, Ganger D, Jensen D. Spontaneous bacterial peritonitis: an update on evaluation, management, and prevention. Am J Med. 1994. 97:169–175.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download