Abstract
We report a case of toxic epidermal necrolysis with ocular involvement following vaccination for hemorrhagic fever with renal syndrome. A healthy 20-year-old male soldier presented with confluent purpuric and erythematous dusky red macules evolving to flaccid blister and epidermal detachment on the whole body with conjunctival injection. The patient had no antecedent medical or surgical conditions except for two doses of hemorrhagic fever with renal syndrome vaccination. With supportive care, skin lesions were improved. Ophthalmic examinations revealed conjunctival injection with epithelial defects in both eyes. Ocular complications were resolved after amniotic membrane transplantation. Toxic epidermal necrolysis may be considered as a possible complication of hemorrhagic fever with renal syndrome vaccination.
Toxic epidermal necrolysis (TEN) is an acute and severe skin reaction characterized by widespread erythema, blisters, and sheet-like skin loss, often associated with a systemic toxic condition and mucous membrane involvement including the ocular tissues.1-6 TEN is assumed to be related to hypersensitivity reactions to drugs and infections.1-6 Vaccination has been reported as a rare triggering factor for erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and TEN.7-10 To date, there is no report of EM/SJS/TEN related to hemorrhagic fever with renal syndrome (HFRS) vaccination. We report herein a case of TEN with ocular involvement following vaccination for HFRS.
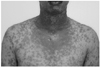
A healthy 20-year-old male soldier presented with confluent purpuric and erythematous dusky red macules evolving to flaccid blister and epidermal detachment on the whole body with extensive erosions and necroses of the oral mucosa (Fig. 1). Nikolsky sign was positive on erythematous zones. The patient had no antecedent medical or surgical conditions or medications including NSAID except for two doses of HFRS vaccination (Hantavax®; Korea Green Cross, Seoul, Korea) at one month interval. The first symptom of bilateral conjunctival injection and tearing developed one day after second vaccination. And then, other symptoms such as swelling of lips, malaise, headache, pharyngeal irritation, and conjunctival itching sense developed. However, there were no symptoms such as fever, myalgia, nausea, diarrhea, and arthralgia. Skin vesicles and papules quickly developed and became widespread involving oral mucosa, neck, and upper extremities followed by dissemination to the trunk and legs. The palms and soles were unaffected. Laboratory findings including complete blood cell count, electrolyte/BUN/Cr analysis, liver function test, urine analysis, and tests for autoimmune diseases (anti-nuclear antibody, anti-Smith antibody, anti-neutrophil cytoplasmic antibody, and smooth muscle antibody) were within normal range. Because the total area of epidermis detachment was greater than 30% of total body surface, the patient was diagnosed as TEN and high dose systemic corticosteroid and prophylactic antibiotics with supportive treatment were administered. Skin lesions improved slowly with these methods.
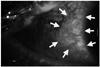
Ophthalmic examinations revealed visual acuity of 20/40 and conjunctival injection with medial, inferior, and temporal conjunctival epithelial defects in both eyes at presentation (Fig. 2). Topical steroid (PredForte®; Allergan, Irvine, CA, USA), antibiotics (Vigamox®; Alcon, Fort Worth, TX, USA), and lubricant ointment (Duratears®; Alcon, Fort Worth, TX, USA) were applied in both eyes. To minimize ocular complication associated with ocular surface inflammation, bilateral amniotic membrane transplantation (AMT) was performed covering whole ocular surface including cornea, lid margins, bulbar and tarsal conjunctivas 5 days after admission. After the surgery, symblepharon rings and therapeutic contact lenses were applied in both eyes. Three weeks after the operation, the ocular complications disappeared, and visual acuity was 20/20 in both eyes without any symblepharon formation.
Various factors have been reported to be associated with TEN. Among them, the most frequent causes were drugs and infections.1-6 Vaccination for human papillomavirus, hepatitis B, smallpox, anthrax, tetanus, mumps, measles, rubella, and influenza has been reported as a rare cause of EM/SJS/TEN.7-10 To date, there has been no published case of TEN related to HFRS vaccination.
HFRS is a life-threatening disease presented by sudden fever, chills, nausea, petechiae, headache, and backache; the most serious aspect of the disease is vascular leakage, acute shock, and renal failure.11-13 Mortality ranges of HFRS have been to be estimated up to 15%.11-13 Approximately 150,000 to 200,000 patients with HFRS are hospitalized each year throughout the world and many of the patients are solders and farmers because HFRS is transmitted by rat.12 In Asia, Hantaan virus and Seoul virus have been identified and known as important causative agents for HFRS in Korea and China.13
The first Hantaan virus vaccine (Hantavax®) was developed from suckling mouse brain and inactivated with 0.05% formalin.12 The recommended immunization schedule of Hantavax® is a series of two doses one month apart with one booster 12 months later.12 From 1990 to 1998, 5,690,000 doses of Hantavax® were used in Korea (1,162,000 doses to soldiers, 20.4%).12 However, little is known about the complications related to Hantavax® vaccination.
Verification of causes of TEN is difficult. Therefore, temporal relationship and absence of other known causal events have been considered as proofs of causal relationship in the previous studies.1-10 Considering the temporal relationship between the development of TEN and vaccination, the HFRS vaccination was the most possible cause in the present case, without any other known causes of TEN including infections and drugs. Bilateral conjunctival injection and tearing developed as the first symptom one day after the second HFRS vaccination followed by whole body skin eruptions in our case, in good accord with the previous studies. Time interval between the administration of the vaccine and EM/SJS/TEN development varied between 1 day and 3 weeks,7-10 and acute conjunctivitis occurred several hours to 4 days before skin eruptions in many cases.5
The mechanism of TEN following HFRS vaccination is not clear. It has been hypothesized that the protein components of vaccine act like keratinocyte-expressed antigens, thus triggering immune reactions.7,12
Ocular involvement of TEN occurs in 50% to 88% of cases and can cause severe complications.1-6 Ocular management includes application of lubricant ointment, antibiotics to prevent infection, steroids to control inflammation, and periodic lysis of symblepharon.14 AMT is known as a recent strategy to suppress inflammation, prevent ulcer formation, and promote healing during the acute stage of TEN, thus preventing sight-threatening cicatrical complications.14 In the present case, ocular complications were recovered after AMT with supportive care with eye drops. The AMT in acute stage of TEN would be an effective strategy to facilitate epithelial healing and reduce inflammation as reported previously.14
In conclusion, we experienced the first case of TEN with ocular involvement developed after HFRS vaccination. TEN may be considered as a possible complication of HFRS vaccination.
Figures and Tables
ACKNOWLEDGEMENTS
The study was supported by the Korean Military Medical Research Project from the ROK Defense Ministry. The funding sources had no role in study design, data collection and analysis, manuscript preparation, or the decision to submit the manuscript for publication.
References
1. Power WJ, Ghoraishi M, Merayo-Lloves J, Neves RA, Foster CS. Analysis of the acute ophthalmic manifestations of the erythema multiforme/Stevens-Johnson syndrome/toxic epidermal necrolysis disease spectrum. Ophthalmology. 1995. 102:1669–1676.

2. Revuz J, Penso D, Roujeau JC, Guillaume JC, Payne CR, Wechsler J, et al. Toxic epidermal necrolysis. Clinical findings and prognosis factors in 87 patients. Arch Dermatol. 1987. 123:1160–1165.

3. Chang YS, Huang FC, Tseng SH, Hsu CK, Ho CL, Sheu HM. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis: acute ocular manifestations, causes, and management. Cornea. 2007. 26:123–129.

4. López-García JS, Rivas Jara L, García-Lozano CI, Conesa E, de Juan IE, Murube del Castillo J. Ocular features and histopathologic changes during follow-up of toxic epidermal necrolysis. Ophthalmology. 2011. 118:265–271.

5. Sotozono C, Ueta M, Koizumi N, Inatomi T, Shirakata Y, Ikezawa Z, et al. Diagnosis and treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis with ocular complications. Ophthalmology. 2009. 116:685–690.

6. Gueudry J, Roujeau JC, Binaghi M, Soubrane G, Muraine M. Risk factors for the development of ocular complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. Arch Dermatol. 2009. 145:157–162.

7. Katoulis AC, Liakou A, Bozi E, Theodorakis M, Alevizou A, Zafeiraki A, et al. Erythema multiforme following vaccination for human papillomavirus. Dermatology. 2010. 220:60–62.

8. Chopra A, Drage LA, Hanson EM, Touchet NL. Stevens-Johnson syndrome after immunization with smallpox, anthrax, and tetanus vaccines. Mayo Clin Proc. 2004. 79:1193–1196.

9. Kaur S, Handa S. Erythema multiforme following vaccination in an infant. Indian J Dermatol Venereol Leprol. 2008. 74:251–253.

10. Ball R, Ball LK, Wise RP, Braun MM, Beeler JA, Salive ME. Stevens-Johnson syndrome and toxic epidermal necrolysis after vaccination: reports to the vaccine adverse event reporting system. Pediatr Infect Dis J. 2001. 20:219–223.

12. Sohn YM, Rho HO, Park MS, Kim JS, Summers PL. Primary humoral immune responses to formalin inactivated hemorrhagic fever with renal syndrome vaccine (Hantavax): consideration of active immunization in South Korea. Yonsei Med J. 2001. 42:278–284.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download