Abstract
We report herein a case successful endovascular treatment with a stent-graft of a rare case of rapidly growing mycotic aneurysm of the left common carotid artery due to acute bacterial endocarditis after eradication of the infection. Infected mycotic aneurysms of the peripheral vasculature have been considered as a contraindication for stent-graft implantation because of the possibility of microorganism spreading to the stent-graft; however, if there is evidence of complete eradication of microorganism and surgery is not an option, stent-graft implantation can be an effective and safe treatment modality for exclusion of the mycotic aneurysm.
Mycotic aneurysm, an infection-related false aneurysm, is a very rare entity in the common carotid artery (CCA). While most reported cases developed following carotid endarterectomy and traumatic dissection of the cervical carotid artery, mycotic aneurysm in the CCA secondary to acute bacterial endocarditis has extremely rarely been reported;1-3 they are most commonly located in the intracranial internal carotid artery (ICA) and the cerebral artery.4 Traditional surgical repair of carotid mycotic aneurysms, which is demanding because of the potential for rupture and distal embolization of thrombus in the mycotic aneurysm, is associated with relatively high morbidity and mortality.5
We describe herein a rare case of a common carotid mycotic aneurysm that developed acutely in the context of bacterial endocarditis and was our successfully treated with a stent-graft.
An 87-year-old woman presented with sudden headache, transcortical sensory aphasia and mild right sided weakness. Initial computed tomography (CT) and magnetic resonance imaging (MRI) of the brain showed an acute hemodynamic cerebral infarction of the left frontal and parietal lobes and a focal severe stenosis of the proximal left ICA. Echocardiography showed no evidence of abnormalities of the cardiac valves or of left ventricular dysfunction. Three days after admission, cerebral angiography showed a focal severe stenosis (75%) of the proximal left ICA without abnormal findings in either common carotid artery (Fig. 1A and B). However, six days after admission, fever (40℃) suddenly developed with NIH Stroke Scale (NIHSS) score deteriorating from 4 to 10, due to additional aphasia and worsened weakness. Three days after the onset of fever, brain MRI showed no newly developed acute lesion, and echocardiography showed a newly visible 6×5 mm valvular vegetation of the non-coronary cusp of the aortic valve (Fig. 1C). Blood cultures were positive for Staphylococcus aureus 4 days after the onset of fever as the offending microorganism. Intravenous antibiotic therapy was started immediately with a combination of cefazolin and gentamycin. Seven days after antibiotic therapy, the patient was diagnosed with bacterial meningitis, and cerebrospinal fluid (CSF) analysis revealed a white blood cell count of 240/mm3 (leucocytes, 71%; lymphocytes, 29%), red blood cell count of 2900/mm3, glucose concentration: 50 mg/dL, protein concentration: 216 mg/dL, and pH 8.0. Twenty-six days after admission, chest CT, which was performed with the suspicion of the Lt. pleural effusion as the cause of the fever, showed an incidental 22×20 mm thin walled saccular vascular structure located 2 cm distal to the origin of the left CCA suggesting a mycotic aneurysm because of acute development, presence of ongoing fever and inflammatory infiltration around the saccular vascular structure (Fig. 1D). Three days after the chest CT, the patient's fever and the results of repeated CSF analysis and blood culture were found to be normalized. Because of the latter evidence for complete eradication of septicemia and the patient's old age and cardio-neurologic risk factors for surgery, the patient was immediately subjected to an interventional procedure to treat the mycotic aneurysm. Thoracic aortography showed a saccular aneurysm in the proximal left CCA (Fig. 1E). A 10 mm×5 cm polytetrafluoroethylene covered stent-graft (Hercules Vascular: S&G Biotech, Seongnam, Korea) was deployed within the proximal left CCA across the wide neck of the aneurysm (Fig. 1F). The final thoracic aortography revealed a complete exclusion of the aneurysm and a widely patent implanted stent-graft (Fig. 1G). During the 2 weeks after the procedure, intravenous antibiotic with cefazolin and anti-pletelet therapy with 325 mg aspirin and 75 mg of clopidogrel were continued, and patient's neurologic symptoms gradually improved. The final transesophageal echocardiography showed a subtly improved vegetation, compared with previous echocardiography.
After 2 weeks of additional anti-pletelet treatment with the same medication regimen, the patient was discharged without neurological deficits.
Follow-up chest CT 12 months after the procedure showed the aneurysm completely excluded and a widely patent stent-graft (Fig. 1H). The patient remained asymptomatic during the 12-months follow-up period on continued anti-pletelet treatment with 100 mg aspirin and 75 mg of clopidogrel.
First described by Osler in 1885, mycotic aneurysms show a distinct infectious pathologic process in their walls in resected or autopsy specimens. Nowadays, this term is used for false aneurysms related to any infection, whether intravascular or systemic, such as infective endocarditis or trauma-related such as arterial puncture or penetrating injuries, and locoregional infection such as meningitis, cervical adenitis, and dental abscess.5,6
Mycotic aneurysm associated with infectious endocarditis is quite rare and most commonly located in the intracranial vasculature, especially in bifurcation areas. Involvement of the extracranial carotid artery is extremely rare relative to ICA involvement. The pathogenesis of mycotic aneurysm includes: 1) septic emboli, which tend to lodge at branching points of distal vessels or reach the vasa vasorum to infect and weaken the arterial wall; 2) contiguous inflammatory processes, which cause an infective aneurysm by involving the periarterial lymphatics and the vasa vasorum, such as in meningitis; and 3) iatrogenic vascular manipulation with postoperative or vascular trauma precipitating infection in the arterial wall.
Most mycotic aneurysms of the CCA reported in the English literature are iatrogenic after carotid endarterectomy and trauma, and reports on catheter manipulation-related mycotic aneurysm and its incidence are extremely rare.7,8
Various surgical procedures have been described for the treatment of mycotic aneurysms in the extracranial carotid artery; however, stroke risk and mortality rates remain high at 9-10%.9,10
Although the efficacy and safety of treatment with a covered stent for a carotid mycotic aneurysm has not yet been well established, active bacterial endocarditis should be resolved or brought under control by antibiotic therapy before the procedure. Furthermore, the long-term patency and risk of thromboembolism of the stent-grafts in CCA remain to be determined, especially in the context of arterial infection and contraindication for anticoagulation treatment after the procedure. Published reports include only short-term follow-up data.
The successful result of endovascular treatment in the case described herein indicates that stent-graft placement in the setting of bacterial endocartitis may be a safe and effective treatment option when used in combination with prolonged antibiotic and anticoagulant therapy.
Figures and Tables
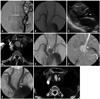 | Fig. 1Bacterial endocarditis and mycotic aneurysm of the left common carotid artery in an 87-year-old woman with acute cerebral infarction. (A) Transfemoral cerebral angiography showing focal severe stenosis (up to 75%) of the proximal left ICA (arrow) and transient vascular spasm in the petrous ICA (arrowheads). (B) Thoracic aortography showing no evidence of stenosis or aneurysm of the proximal common carotid artery. (C) Echocardiography showing a newly visible 6×5 mm valvular vegetation of the non-coronary cusp of the aortic valve (arrow), which was not present in preprocedural echocardiography. (D) Chest CT 26 days after admission showing an incidental 22 mm×20 mm saccular aneurysm 2 cm distal to the origin of the left CCA (arrow). (E) Thoracic aortography three days after chest CT showing a saccular aneurysm in the proximal left CCA (arrow) with mass effect to the lumen of the adjacent proximal CCA (arrowhead). (F) The roadmap image of the left common carotid artery during stent insertion showing proper advancement of an undeployed 10 mm×5 cm polytetrafluoroethylene covered stent-graft across the wide neck of the aneurysm in the proximal left common carotid artery (arrows). (G) The final thoracic aortography after stent-graft implantation revealing complete exclusion of the aneurysm and a widely patent implanted stent-graft (arrows). (H) Follow-up chest CT 12 months after the procedure showing a completely excluded aneurysm, thrombosis within the aneurismal lumen (arrow), and a widely patent stent-graft (arrowheads). ICA, internal carotid artery; CT, computed tomography; CCA, common carotid artery. |
References
1. Jebara VA, Acar C, Dervanian P, Chachques JC, Bischoff N, Uva MS, et al. Mycotic aneurysms of the carotid arteries--case report and review of the literature. J Vasc Surg. 1991. 14:215–219.

2. Hubaut JJ, Albat B, Frapier JM, Chaptal PA. Mycotic aneurysm of the extracranial carotid artery: an uncommon complication of bacterial endocarditis. Ann Vasc Surg. 1997. 11:634–636.

3. Angle N, Dorafshar AH, Ahn SS. Mycotic aneurysm of the internal carotid artery--a case report. Vasc Endovascular Surg. 2003. 37:213–217.
4. Kannoth S, Iyer R, Thomas SV, Furtado SV, Rajesh BJ, Kesavadas C, et al. Intracranial infectious aneurysm: presentation, management and outcome. J Neurol Sci. 2007. 256:3–9.

5. Heyd J, Yinnon AM. Mycotic aneurysm of the external carotid artery. J Cardiovasc Surg (Torino). 1994. 35:329–331.
6. Khalil I, Nawfal G. Mycotic aneurysms of the carotid artery: ligation vs. reconstruction--case report and review of the literature. Eur J Vasc Surg. 1993. 7:588–591.

7. El-Sabrout R, Reul G, Cooley DA. Infected postcarotid endarterectomy pseudoaneurysms: retrospective review of a series. Ann Vasc Surg. 2000. 14:239–247.

8. El-Sabrout R, Cooley DA. Extracranial carotid artery aneurysms: Texas Heart Institute experience. J Vasc Surg. 2000. 31:702–712.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download