The first case of pandemic H1N1/09 in Korea was identified on May 2, 2009. After that time, the number of confirmed cases increased steadily; according to the reports of the Korea Centers for Disease Control and Prevention (KCDC), which operates the Korean emergency response system, a total of 2,417 cases had been reported by August 19, 2009.
1 Beginning August 21, 2009, the KCDC shifted its strategy from containment to mitigation and altered the antiviral agent prescription guidelines to allow clinicians to prescribe antiviral agents to all patients with symptoms of influenza-like illness without a laboratory diagnosis.
2 Moreover, after that time, the KCDC did not conduct individual epidemiologic investigations of confirmed cases. The incidence of influenza-like illness (ILI) and consumption of antiviral agents peaked in early November.
2
The initial literature concerning the pandemic H1N1/09 infection associated the strain with profound morbidity and mortality.
3-
7 However, data on outpatients infected with pandemic H1N1/09 are still lacking. In this study, we describe the clinical characteristics of outpatients with pandemic H1N1/09 during the mitigation period at a tertiary university hospital in Korea.
We conducted a retrospective review of the presentation of outpatients with ILI at Kyung Hee University Hospital between September and December 2009. This study was approved by the IRB. In the vast majority of patients with ILI, pandemic H1N1/09 infection was confirmed by laboratory testing. Written informed consent was obtained from all participants. The department of laboratory medicine at Kyung Hee University Hospital was designated as a quarantine station laboratory and was thus a treatment hub for pandemic H1N1/09. We began laboratory testing for pandemic H1N1/09 on September 1, 2009.
Nose and/or throat swabs were collected from almost all subjects with ILI. Infection with pandemic H1N1/09 was confirmed via real-time reverse transcriptase-polymerase chain reaction (RT-PCR, RealTime Ready influenza A/H1N1 Detection set, Roche Diagnostics GmbH, Mannheim, Germany) and/or multiplex RT-PCR (Multiplex FluA ACE Subtyping, Seegene, Inc., Seoul, Korea).
The KCDC criteria for suspicion of pandemic H1N1/09 infection were cases in which patients suffered from an unexplained fever (defined by an ear temperature ≥37.8℃ or antipyretics within 12 hours before hospital visit) within seven days before visiting the hospital and displayed symptoms such as a cough, sore throat, rhinorrhea, or nasal obstruction.
Statistical analysis was conducted using SPSS Statistics version 12.0 (SPSS, Inc., Chicago, IL, USA). We conducted t-tests for quantitative data, chi-square or Fisher's exact tests for qualitative data, depending on the anticipated cell number, and Cochran Mantel-Haenzel tests for investigating the confounding variable of age. Multivariate logistic regression analyses were employed to evaluate the independent risk factors for pandemic H1N1/09-positive status by backward (wald) stepwise logistic regression models. Only the variables with p<0.1 by univariate analysis were included in the multivariate analysis. Finally, a p-value <0.05 was considered to be indicative of statistical significance. We then calculated sensitivity, specificity, area under the ROC curve (AUC), positive likelihood ratios (PLR), negative likelihood ratios (NLR), positive predictive value (PPV), and negative predictive value (NPV) of the possible diagnostic criteria to identify those that were best for pandemic H1N1/09.
Between September 1 and December 31, confirmed-PCR tests for pandemic H1N1/09 were performed for a total of 7,182 patients (
Fig. 1). Of these, 3,020 (42.0%) were positive. We randomly selected 1,458 individuals (approximately 20%) using SPSS Statistics. Of these, we excluded 399 inpatients, patients without detailed information about the onset of their illness, and those who had been sick for more than seven days. A total of 1,059 outpatients were include in this study, and of these, 586 patients were confirmed to have the pandemic H1N1/09 by laboratory test.
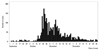
In addition, we investigated the age distribution of pandemic H1N1/09 as well as the positivity rate according to age distribution (
Fig. 2). Approximate numbers of infections peaked in two age groups: the pediatric and adult age groups.
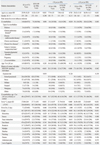
We compared patients with pandemic H1N1/09 and those with ILI, and then compared those groups divided by age (<18 years and ≥18 years) using stratified analysis, Cochran Mantel-Haenzel tests (
Table 1). In the latter cases, there were differences in significant variables for distinguishing characteristics of pandemic H1N1/09 between the two groups (<18 age group, only: sore throat; ≥18 age group, only: age, co-mobidities, risk of age, and myalgia).
Multivariate analysis (
Table 2) of the whole age group, suggested that subjects with confirmed pandemic H1N1/09 were less likely to be older [odds ratio (OR) 0.975, 95% confidence interval (CI) 0.966-0.984,
p<0.001] and to have at least one of the following co-morbidities: age <5 or ≥65 years, a history of contact (OR 0.611, 95% CI 0.464-0.805,
p<0.001), and were also more likely to have a fever of ≥37.8℃ (OR 3.567, 95% CI 2.655-4.792,
p<0.001), cough (OR 2.290, 95% CI 1.692-3.101,
p<0.001), and myalgia (OR 1.599, 95% CI 1.1093-2.223,
p=0.014) than subjects suffering ILI. However, multivariate analysis in the <18 age group, suggested that subjects with pandemic H1N1/09 were more likely to have a fever of ≥37.8℃ (OR 3.142, 95% CI 2.041-4.835,
p<0.001), cough (OR 2.073, 95% CI 1.304-3.296,
p=0.002), and sneeze (OR 2.543, 95% CI 1.148-5.632,
p=0.021) than patients with ILI. In multivariate analysis of the ≥18 age group, a fever of ≥37.8℃ (OR 4.388, 95% CI 2.885-6.674,
p<0.001) and cough (OR 2.677, 95% CI 1.779-4.030,
p<0.001) were more frequent, and older age (OR 0.960, 95% CI 0.945-0.974,
p<0.001) was less frequent in patients with pandemic H1N1/09. Interestingly, in contrast with the results of univariated analyses, the significant variables were not largely different except for sneeze (<18 age group, only) and age (≥18 age group, only), between the two groups.
A previous study
8 reported that those with confirmed pandemic H1N1/09 were more likely to report feverishness, chills, or joint stiffness (OR 3.8, 95% CI 1.6-9.0) and rhinorrhea or nasal congestion (OR 2.5, 95% CI 1.2-5.3), and were also more likely to have ≥38.1℃ fever (OR 6.7, 95% CI 2.7-16.7) compared to those with acute respiratory illness. Moreover, the clinical features of infection with pandemic H1N1/09 are similar to those of seasonal influenza.
9-
11 Subjects with pandemic H1N1/09 influenza tended to have fever, cough, shortness of breath, myalgia, and fatigue, all similar symptoms to those suffering seasonal influenza.
Symptoms of 2009 pandemic H1N1 in children are similar to those of seasonal influenza; for example, fever, cough, sore throat, and myalgia.
7,
10-
11 Other symptoms (such as diarrhea or vomiting) have occasionally been reported in children and in less than 5% of adults during periods of peak seasonal influenza incidence.
12 Another study
10 reported no significant differences in these symptoms between children and adults with pandemic H1N1/09.
Several other studies have suggested that young patients constituted a higher proportion of those positive for pandemic H1N1/09.
2,
4,
5,
13-
15 This may have been due to an outbreak of pandemic H1N1/09 in preschools or schools. In the present study, subjects with pandemic H1N1/09 with a history of contact with other pandemic H1N1/09-infected individuals accounted for 16.6% of the relevant group. Of those subjects <18 years of age, 12.8% had a history of contact with other infected subjects; these exposures occurred mainly at school (60.0%) and at home (34.3%).
Unlike those suffering from ILI, all subjects with confirmed pandemic H1N1/09 fulfilled at least one of the following conditions: age <5 or ≥65 years, or a history of contact (48.4% vs. 34.5%,
p<0.001) identified via logistic regression analysis. These findings differ from those of other studies.
9,
10 The difference may be attributed, at least in part, to the time of investigation. This study was conducted during the mitigation period, between September and December 2009. Therefore, active outbreak control strategies such as school closures, mass vaccination, and an anti-viral drug regimen had been applied, and sufficient health information had been provided to the public.
We assessed validation of sensitivity, specificity, AUC, PLR, NLR, PPV, and NPV for key variables significantly associated with pandemic H1N1/09 infection (
Table 3). However, considering the sensitivity, PLR, and PPV together, it was difficult to determine the best criteria for clinical diagnosis of pandemic H1N1/09 in outpatients, likely due to its non-specific presentation. We found no clear criteria for having a higher pretest probability (PLR ≥5 and NLR ≤ 0.2) in this study, especially considering the PLR and NLR from a previous study.
16
In the whole age group, the sensitivity of "fever (≥37.8℃) plus cough" (41.03%, 95% CI 37.1-45.1) was lower than that of the KCDC criteria, whereas the PLR (3.55, 95% CI 3.2-3.9) and PPV (81.6, 95% CI 76.7-85.9) of this criterion were higher than those of KCDC criteria. Interestingly, most clinical physicians' determinations of pandemic H1N1/09 were not compliant with the KCDC criteria, so the accuracy and predictability insofar as sensitivity, specificity, PLR, NLR, PPV, or NPV were different from those of the KCDC criteria. This indicates that the KCDC criteria were not actually sufficient for clinicians to distinguish pandemic H1N1/09 from ILI.
In the <18 age group, the criterion of "fever (≥37.8℃) plus cough or sneeze" was the most effective (sensitivity: 42.07%, 95% CI 36.3-48.0; PLR: 2.52, 95% CI 2.2-2.9; PPV; 80.3, 95% CI 73.0-86.3). In the ≥18 age group, the criteria of "fever (≥37.8℃) plus cough" was the most effective (sensitivity: 41.36%, 95% CI 35.7-47.2; PLR: 4.98, 95% CI 4.3-5.7; PPV; 83.6, 95% CI 55.8-65.1).
A previous study
17 suggested the combination of cough plus fever or myalgia (sensitivity 73.95, PPV 66.4%) as the diagnostic criteria of pandemic H1N1/09 infection because of its higher sensitivity and PPV than those of the KCDC, WHO, or ILI criteria. Another previous study
8 reported that the absence of leukocytosis combined with the WHO criteria was the best criteria (PLR 7.8, 95% CI 3.5-17.5) in diagnosing pandemic H1N1/09. However, because there was a notable absence of data from other laboratory tests and imaging examinations in that study, we could not assess these variables to diagnose pandemic H1N1/09. Our results suggested that there was a limit to the ability to distinguish pandemic H1N1/09 infection from ILI by only symptoms or signs, because of somewhat lower sensitivity, PLR, and PPV. Therefore, we propose that the use of laboratory confirmatory testing is necessary to diagnose infection with pandemic H1N1/09.
The KCDC reported that the incidence of ILI and consumption of antiviral agents peaked in early November. Following vaccination of health care workers on October 27, 2009, approximately 12 million people were vaccinated in Korea.
2 One previous study
18 reported that this rapid vaccination was the most important factor in controlling the spread of pandemic influenza in Korea. However, our study could not include an evaluative vaccination history because most medical charts were missing these data. Additionally, this study is limited by its retrospective design and single-center origin. However, it is worth noting that large numbers of subjects from a single center were analyzed, and these data should prove useful to local influenza control centers.
In conclusion, our analysis suggested that the clinical characteristics of infection with pandemic H1N1/09 are indistinguishable from those of influenza-like illness in many respects. Moreover, the accuracy and predictability of criteria that included only symptoms or signs were not sufficient to diagnose pandemic H1N1/09 infection. Therefore, a combination of clinical diagnosis and confirmatory laboratory tests is required for accurate diagnosis of pandemic H1N1/09 infection.







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download