Abstract
Purpose
During stimulated in vitro fertilization (IVF) cycle, up to 30% of the recovered oocytes are immature ones which have poor fertilization capacity; however, the precise influencing factors are largely unknown. Here, we analyzed the association of oocyte immaturity with woman's age in IVF cycles stimulated by single regimen.
Materials and Methods
A total of one-hundred ninety five IVF cycles stimulated by recombinant FSH and GnRH antagonist protocol between 2003 and 2009 were analyzed retrospectively. The mean age of women was 34.2±4.0 (26-45 years). After triggering by exogenous hCG, an ultrasound-guided retrieval of oocytes was performed 35-36 hours later. All clinical data were stratified by woman's age; group I: ≤30 (n=36), II: 31-35 (n=83), III: 36-40 (n=57), and IV: ≥41 (n=19).
During stimulation of in vitro fertilization (IVF) cycle, up to 30% of the recovered oocytes are immature ones.1-7 Reducing yield of immature oocytes is an another important aspect in stimulated IVF cycles because they usually have lower maturation capacity and seldom yield transferable embryos.6 In clinical practice, improving developmental competence of these rescued oocytes in vitro would be useful for women who yield only immature oocytes or show total fertilization failure of in vivo matured oocytes in stimulated IVF cycles.8-12
It is unclear why oocytes remain immature despite adequate ovarian stimulation. The immature oocytes may originate from small antral follicles at the time of oocyte retrieval or from large preovulatory follicles which do not respond to hCG appropriately. During ovarian stimulation, the oocyte population at the time of hCG may be heterogeneous, leading to retrieval of oocytes at different stages of maturation.
Currently, the mechanisms and the affecting factors to yield immature oocyte in stimulated IVF cycles are poorly understood. A high rate of oocyte immaturity was reported to be associated with young female age,5 the use of GnRH antagonist rather than GnRH agonist,4 and urinary hCG as a triggering agent rather than recombinant form.7 When all IVF cycles stratified were by two patient age groups (<35 or ≥35 years), the incidence of abnormally higher oocyte immaturity (i.e. the cycles retrieving >50% immature oocytes) was found to be significantly higher in <35 group than ≥35 group (8.5% vs. 1.4%).5 It has also been reported that urinary gonadotropin increases the yield of mature oocytes in patients with a previous cycle having a high incidence of oocyte immaturity by using recombinant FSH.7
We hypothesized that oocyte immaturity would be more common in older women undergoing stimulated IVF cycle because of their closer proximity to ovarian senescence and menopause. In the present study, we investigated the association between woman's age and oocyte immaturity in IVF cycles stimulated by single regimen: i.e. recombinant FSH (rFSH) and GnRH antagonist.
A total of 195 IVF cycles stimulated by rFSH and GnRH antagonist during a period between 2003 and 2009 were selected. We obtained Institutional Review Board approval, although this was a retrospective comparative study. The mean age of women was 34.2±4.0 years with a range of 26-45 years. The infertility factors were identified as the following: tubal (n=56), unexplained (n=54), male (n=29), peritoneal (n=19), age factor (n=16), uterine (n=12), and ovulatory (n=9).
Ovarian hyperstimulation was performed using rFSH (Gonal-F; Serono, Geneva, Switzerland) beginning on day 3 of menstrual cycle. The pituitary was suppressed by flexible multiple-dose protocol of GnRH antagonist (Cetrotide; Serono). Follicular development was monitored with periodic transvaginal ultrasounds and serum levels of estradiol. When dominant follicles averaged 19 mm in diameter, ovulation was triggered by 5,000 IU of urinary hCG (Profasi; Serono) or 250 µg of recombinant hCG (Ovidrel; Serono). An ultrasound-guided retrieval of oocytes was performed 35-36 hours later. The collected cumulus-oocyte complexes were assessed by the presence or absence of a germinal vesicle (GV) or the first polar body (PB) by stereomicroscope (×200). In situation of unclear maturity, the oocytes were sometimes denuded by using 85 IU/mL hyaluronidase (Cook) and mechanical pipetting.
Immature oocytes were defined as oocytes that appeared arrested at either prophase I (oocytes with GV) or metaphase I (evidence of GV breakdown but no PB visible). Immature oocytes were then cultured in commercial IVM medium (Cook-BL; Cook, Brisbane, Australia) supplemented with rFSH 75 mIU/mL (Serono), rhCG 0.5 IU/mL (Serono) and rEGF 10 ng/mL (Invitrogen, Carlsbad, CA, USA). All immature oocytes were cultured in 1 mL each of IVM medium up to 48 hours in an atmosphere of 5% CO2 and 95% air with high humidity. After IVM, they were stripped with 85 IU/mL hyaluronidase and mechanical pipetting until completely denuded of their cumulus cells. If matured, they were then fertilized by conventional insemination as the same method in in vivo matured oocytes. Normal fertilization was confirmed when two distinct pronuclei were present 16-18 hours later. Embryo culture was performed as described previously.6
All data in the present study were analyzed using SPSS (Windows version 12.0). The Chi square test was used to compare two proportions. Since all data from four age groups showed a normal distribution, we used the parametric ANOVA test to compare the means within four groups; when a significant difference identified, the Scheffe Post Hoc test was applied. A p-value of <0.05 (two-tailed) was always considered statistically significant.
As shown in Table 1, causes of infertility, mean body mass index, and basal FSH level were not different between the four age groups. The mean antral follicle count was lowest in group IV, which was significantly different from that of group II. The dose of exogenous gonadotropins (ampoules) tended to be higher in group IV, but serum estradiol level at triggering day was similar in four groups. The fertilization rate of in vivo matured oocytes was similar among the four groups. The number of transferred embryos was lowest in group IV, and accordingly, the clinical pregnancy rate was significantly lower in group IV when compared with other age groups.
The mean number of total retrieved oocyte, as well as the number of immature oocyte, tended to decrease as increasing age (Fig. 1). The resultant mean % of immature oocyte tended to be decreased as increasing age, however, this phenomenon was observed only in women aged 40 years or less (37.9% in group I, 32.4% in group II, and 23.3% in group III, p>0.05). The mean % of immature oocyte was the highest in group IV with statistical significance, compared with group II and III. More than half of the retrieved oocytes were immature ones in women aged 40 years or more.
In the present study, we attempted to investigated the relation between patient's age and the rate of immature oocytes in IVF cycles. The oocyte immaturity was the highest in women aged 40 years or more; however, oocyte immaturity tended to rather decrease as increasing age in women aged 40 years or less. In contrary to our hypothesis, the rate of immature oocytes was not strictly proportional to women's age. This observation in part is consistent with the previous study which showed that oocyte immaturity was more frequent in <35-year group than ≥35-year group.5
It is highly likely that women aged 40 years or more have higher oocyte immaturity during stimulated IVF cycle because ovarian senescence progresses in those group. However, relatively higher oocyte immaturity was also noted in women aged 30 years or less, and the reason is still unclear. Young patients in general are high responders, therefore, they might have higher heterogeneity of follicular development. In the same context, they might have higher chance not to respond to usual dosage of hCG. Higher oocyte immaturity in young women would be a reflection of their high follicular response, not attributing to intrinsic oocyte factors. This explanation seems to be highly possible because the young women in our study yielded similar number of transferred embryos and had a higher clinical and ongoing pregnancy rate despite higher oocyte immaturity.
It was anecdotally reported that 27-year-old Turner mosaicism showed an excellent ovarian response to stimulation during several IVF cycles, but a high proportion (80%) of immature oocytes was noted.13 Since young woman with Turner mosaicism is associated with impending premature ovarian failure, it can be assumed that infertile women who are impending ovarian failure may produce a large proportion of immature oocytes.
When high oocyte immaturity is noted in stimulated IVF cycle, extending duration of triggering has been recommended.14 In order to extend the hCG to retrieval interval, transvaginal oocyte retrieval could be scheduled 41 hours post-hCG instead of conventional 35 hours.
Alternately, change of ovarian stimulation regimen has been suggested to be beneficial in patients with such a high proportion of immature oocyte;7 in a cohort of patients with a high rate of oocyte immaturity during a cycle stimulated with FSH only, the addition of LH in a subsequent cycle increased the yield of both mature oocytes and excellent-quality embryos. These authors suggest that the high rate of oocyte immaturity in patients stimulated with FSH alone may be due to relative deficiency of LH activity; however the requirement of LH for adequate development and maturation of ovarian follicle remains unclear. In the same context, the superiority of HMG in terms of oocyte maturity is not conclusive. Two randomized trials denoted that oocyte maturity was similar between hCG-containing highly purified HMG and rFSH; 69% vs. 67.4%,15 and 71.2% vs. 70.8%.16 Urinary HMG also yielded a similar rate of mature oocyte compared with rFSH; 89.3% vs. 86.2%.17 Even significantly less mature oocyte was reported by using urinary HMG compared with highly purified FSH; 80.6% vs. 88.8%.18 Therefore, currently available evidence does not support the superiority of gonadotropins including LH content, in terms of oocyte maturity.
GnRH antagonist protocol appears to have a better chance to obtain immature oocytes compared with GnRH agonist protocol.4 This may be due to greater heterogeneity in follicle development in patients receiving antagonist protocol.19 In the present study, we selected single stimulation regimen (rFSH only with GnRH antagonist protocol) to eliminate possible confounding effect from variable regimens.
In conclusion, the rate of immature oocyte was not proportional to woman's age; it was the highest in women ≥41 years, followed by young women ≤30 years. Further researches are needed regarding the identification of influencing factors and the mechanisms for oocyte immaturity despite adequate ovarian stimulation. Furthermore, efforts should be continued to reduce its incidence in stimulated IVF cycle.
Figures and Tables
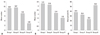 | Fig. 1(A) The mean number of total retrieved oocytes (group I: ≤30 years old, II: 31-35 years, III: 36-40 years, IV: ≥41 years) (I-IV: p=0.001, II-IV: p=0.000, III-IV: p=0.048, ANOVA test with Scheffe's post-hoc test). (B) The mean number of immature oocytes (I-III: p=0.044, I-IV: p=0.008, II-IV: p=0.045). (C) The mean percentage of immature oocytes (II-IV: p=0.049, III-IV: p=0.002). |
References
1. Mandelbaum J, Junca AM, Belaisch-Allart J, Salat-Baroux J, Plachot M, Antoine JM, et al. [Oocyte maturation and intracytoplasmic sperm injection]. Contracept Fertil Sex. 1996. 24:534–538.
2. Cha KY, Chian RC. Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update. 1998. 4:103–120.

3. De Vos A, Van de Velde H, Joris H, Van Steirteghem A. In-vitro matured metaphase-I oocytes have a lower fertilization rate but similar embryo quality as mature metaphase-II oocytes after intracytoplasmic sperm injection. Hum Reprod. 1999. 14:1859–1863.

4. Nogueira D, Friedler S, Schachter M, Raziel A, Ron-El R, Smitz J. Oocyte maturity and preimplantation development in relation to follicle diameter in gonadotropin-releasing hormone agonist or antagonist treatments. Fertil Steril. 2006. 85:578–583.

5. Moore AK, Arny MJ, Lynch K, Grow DR. Oocyte maturation arrest more common in younger patients undergoing IVF/ICSI. Fertil Steril. 2007. 88:Suppl 1. S271.

6. Jee BC, Han SH, Moon JH, Suh CS, Kim SH. Seoul National University College of Medicine Assisted Reproductive Technology (SMART) Study Group. Influence of well defined protein source on in vitro maturation of human oocyte: human follicular fluid versus human serum albumin. Fertil Steril. 2008. 89:348–352.

7. Huddleston HG, Jackson KV, Doyle JO, Racowsky C. hMG increases the yield of mature oocytes and excellent-quality embryos in patients with a previous cycle having a high incidence of oocyte immaturity. Fertil Steril. 2009. 92:946–949.

8. Kim BK, Lee SC, Kim KJ, Han CH, Kim JH. In vitro maturation, fertilization, and development of human germinal vesicle oocytes collected from stimulated cycles. Fertil Steril. 2000. 74:1153–1158.

10. Chian RC, Buckett WM, Abdul Jalil AK, Son WY, Sylvestre C, Rao D, et al. Natural-cycle in vitro fertilization combined with in vitro maturation of immature oocytes is a potential approach in infertility treatment. Fertil Steril. 2004. 82:1675–1678.

11. Jurema MW, Nogueira D. In vitro maturation of human oocytes for assisted reproduction. Fertil Steril. 2006. 86:1277–1291.

12. Oktay K, Demirtas E, Son WY, Lostritto K, Chian RC, Tan SL. In vitro maturation of germinal vesicle oocytes recovered after premature luteinizing hormone surge: description of a novel approach to fertility preservation. Fertil Steril. 2008. 89:228.e19–228.e22.

13. Moore AK, Lynch K, Arny MJ, Grow DR. Turner mosaicism (45,X/46,XX) diagnosed in a young woman subsequent to low oocyte maturity and failed ICSI. Fertil Steril. 2008. 90:2012.e13–2012.e15.

14. Levran D, Farhi J, Nahum H, Glezerman M, Weissman A. Maturation arrest of human oocytes as a cause of infertility: case report. Hum Reprod. 2002. 17:1604–1609.

15. Bosch E, Vidal C, Labarta E, Simon C, Remohi J, Pellicer A. Highly purified hMG versus recombinant FSH in ovarian hyperstimulation with GnRH antagonists--a randomized study. Hum Reprod. 2008. 23:2346–2351.

16. Baker VL, Fujimoto VY, Kettel LM, Adamson GD, Hoehler F, Jones CE, et al. Clinical efficacy of highly purified urinary FSH versus recombinant FSH in volunteers undergoing controlled ovarian stimulation for in vitro fertilization: a randomized, multicenter, investigator-blind trial. Fertil Steril. 2009. 91:1005–1011.

17. Ng EH, Lau EY, Yeung WS, Ho PC. HMG is as good as recombinant human FSH in terms of oocyte and embryo quality: a prospective randomized trial. Hum Reprod. 2001. 16:319–325.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download