Abstract
Purpose
Minimally invasive management of small renal tumors has become more common. We compared the results of partial nephrectomy by video-assisted minilaparotomy surgery (VAMS), open, and laparoscopic techniques.
Materials and Methods
We retrospectively compared clinicopathological, oncological, and functional outcomes in 271 patients who underwent partial nephrectomy for renal tumors at one institution from 1993 to 2007; including 138 by VAMS, 102 by open, and 31 by laparoscopic technique.
Results
Mean follow-up was 47.7±29.1 months. No statistically significant differences in the three groups were found in tumor size, tumor location, estimated blood loss, complication rate, preoperative glomerular filtration rate (GFR), and GFR at last follow-up. Ischemic time was shorter in the open (26.9 min) and VAMS (29.3 min) groups than in the laparoscopic group (31.0 min, p=0.021). Time to normal diet and hospital stay were shorter in the VAMS (1.8 days and 5.4 days) and laparoscopic (1.8 days and 4.7 days) groups than in the open group (2.4 days and 7.3 days, p=0.036 and p<0.001, respectively). Of 180 patients with cancer, positive surgical margins occurred in 2 of 82 patients (2.4%) in the VAMS group, none of 75 patients in the open group, and 3 of 23 patients (13.0%) in the laparoscopic group (p=0.084). In the VAMS, open, and laparoscopic groups, 5-year disease-free survival was 94.8%, 95.8%, and 90.3% (p=0.485), and 5-year cancer-specific survival was 96.3%, 98.6%, and 100%, respectively (p=0.452).
At present, partial nephrectomy is widely accepted and performed as a standard surgical treatment for small renal tumors. As more renal tumors are detected at a small size, interest is widespread in developing various minimally invasive nephron-sparing techniques to minimize postoperative morbidity. Recently, many studies have reported purely laparoscopic and hand-assisted techniques for partial nephrectomy. However, the absence of a standardized method for achieving renal parenchymal hypothermia and technical difficulties in suture closure of the parenchyma and collecting system present obstacles that are yet to be completely overcome. In the laparoscopic retroperitoneal approach, surgery is technically challenging because of lack of surgical space. Such observations prompted us to apply the video-assisted nephrectomy through a minilaparotomy incision to the treatment of incidentally discovered, small, solitary tumors,1-2 and we reported that donor nephrectomy using video-assisted minilaparotomy surgery (VAMS) provides less pain and better cosmesis compared to open donor nephrectomy.3 We have so far performed approximately 1,000 cases using the technique of VAMS to treat renal disease.
In the present study, we examined the clinical, pathological, oncological, and functional outcomes after partial nephrectomy via VAMS compared to standard open and laparoscopic techniques, all of which were performed at a single institution.
We retrospectively reviewed the records of 271 patients who underwent partial nephrectomy for renal masses at our institution from 1993 to 2007. This study was approved by the Institutional Review Board. Among these patients, 138 underwent partial nephrectomy via VAMS, 102 by open technique, and 31 by laparoscopic technique. The approach was based on the preference of the surgeons and patients. Two surgeons performed the VAMS technique, two different surgeons performed the open technique, and another surgeon performed the laparoscopic technique. Tumors were defined as exophytic if the tumor extended more than 60% off the surface of the kidney, endophytic if the tumor extended less than 40%, and mesophytic if the lesion extended between 40% and 60% off the surface of the kidney on CT. Hilar lesions were defined as those located within 5 mm of the renal hilar great vessels. The cases were compared with regard to clinical parameters, operative data, pathological parameters, oncological outcomes, and renal functional outcomes. Glomerular filtration rate (GFR) was measured by using the four-variable Modification of Diet in Renal Disease formula [GFR=186.3×Pcr-1.154×age-0.203×0.742 (if female)].4 After partial nephrectomy, specimens were examined for surgical margins, tumor size, and histological subtype, and the Fuhrman nuclear grade was determined. A positive surgical margin was defined as tumor cells present at the inked margin on pathological review. Follow-up care consisted of physical examination, laboratory evaluation, chest radiography, and abdominal computerized tomography. These procedures were performed semiannually for the first two years and annually thereafter. Complications were listed in a standardized format using the Clavien-Dindo classification of surgical complications.5
The technique used for VAMS partial nephrectomy was similar in all cases. A 6-cm to 7-cm transverse skin incision was made anteriorly from the costal margin corresponding to the level of the 10th rib. We obtained sufficient operative space around the kidney using a specially designed self-retaining retractor (Thompson Surgical Inc., Traverse, MI, USA) (Fig. 1A). The operative field was observed through the monitor or by direct vision. A laparoscopic retrieval sac (LapSac®, Sejong, Korea) was introduced through the minilaparotomy incision and placed around the kidney (Fig. 1B). Laparoscopic bulldog clamps were applied to the renal artery and vein (Fig. 1C). The blind end of the LapSac® was opened with scissors and packed with finely ground ice slush for renal parenchymal hypothermia (Fig. 1D). The tumor was excised using a surgical knife. Any openings in the collecting system and blood vessels were meticulously closed in a watertight manner with 4-0 Vicryl. Monosyn 1-0 sutures were used to approximate the remaining renal parenchyma.
The previously described transperitoneal laparoscopic technique was performed under warm ischemia conditions.6 The open technique was performed with the patient in a full-flank position via an anterior subcostal incision below the 11th rib. The renal artery was clamped using a bulldog clamp, and regional hypothermia was instituted or omitted at the surgeon's discretion.
Data are presented as number (percentage) or mean±standard deviation. Chi-square and analysis of variance tests were used to compare qualitative and quantitative variables, respectively. Disease-free and cancer-specific survival was calculated using life tables and the Kaplan-Meier method with log-rank test statistics. All p values were two-sided, and p<0.05 was considered statistically significant. All data analysis was processed using SPSS statistical software (Statistical Product and Services Solutions, version 12.0, SPSS Inc., Chicago, IL, USA).
The clinicopathological and operative data of 271 patients who underwent VAMS, open, and laparoscopic partial nephrectomy are listed in Table 1. The proportion of women was higher in the VAMS than in the other groups; however, no other statistically significant differences in baseline characteristics were found among the three groups. Among 271 patients, 18 patients (6.6%) had a single kidney at operation, and partial nephrectomy was performed by VAMS technique in 12 patients and by open technique in six patients. Among 18 patients, 16 patients previously underwent nephrectomy before partial nephrectomy; 11 (61.1%) for renal cell carcinoma, 4 (22.2%) for benign renal disease, and one (5.6%) for urothelial carcinoma. Two patients had a unilateral single kidney due to renal agenesis.
Table 1 shows the surgical and functional outcomes. No significant difference was found in estimated blood loss, tumor location, tumor characteristics, need for transfusion, complications, and tumor size. Ischemic time and operative time were shorter in the open group compared to the VAMS and laparoscopic groups. Length of stay was shorter in the VAMS than the open group, but was not statistically different from the laparoscopic group. The overall complication rate was 15.9%. The complication rate was similar among the VAMS, open, and laparoscopic groups. Complications were categorized as grade 1 (hematuria, n=1), grade 2 (transfusion, n=17; infection, n=1; and hematoma, n=1), grade 3 (embolization, n=2), and grade 4 (sepsis, n=1) in the VAMS group. In the open group, there were grade 2 (transfusion, n=13; infection, n=1; and hematoma, n=1), and grade 4 (acute myocardial infarct, n=1) complications. In the laparoscopic group, there were grade 2 (transfusion, n=2 and hematoma, n=1) and grade 3 (bowel injury, n=1) complications. There were no differences in operative time, ischemic time, blood loss, hospital length of stay, or postoperative renal function between the group of 12 patients who underwent VAMS technique and 6 patients who had the open technique with a single kidney (data not shown). Preoperative GFR was similar among the three groups. After a follow-up of 47.7±29.1 months, GFR did not differ among the three groups.
Table 2 compared characteristics of renal cell carcinoma and oncologic outcomes by operative method. The most common pathological stage among the three groups was pT1aN0M0. However, 12 patients (14.6%) had pT1b lesions, and 2 patients (2.4%) had metastatic lesions at partial nephrectomy in the VAMS group. Recurrences were found in all three groups, including 3 patients in the VAMS group (3.7%); 3 patients in the open group (4.0%); and 2 patients in the laparoscopic group (8.7%; p=0.397). Five-year disease-free survival rates were 94.8% in the VAMS group, 95.8% in the open group, and 90.3% in the laparoscopic group, according to Kaplan-Meier analysis with log-rank test (p=0.485) (Fig. 2). Five-year cancer-specific survival rates was 96.3% in the VAMS group, 98.6% in the open group, and 100% in the laparoscopic group, according to Kaplan-Meier analysis using the log-rank test (p=0.452).
Since the number of patients diagnosed with small localized renal tumors has been increasing considerably, the trend of kidney surgery for renal masses is shifting towards minimally invasive surgery. With the evolution of laparoscopic renal surgery, the laparoscopic approach had also been used in nephron-sparing surgery. However, despite the advantages of improved cosmesis, decreased patient morbidity, and faster postoperative recovery, laparoscopic partial nephrectomy has been hindered by difficulties in renal hemostasis, renal hypothermia, and suture repair of renal parenchymal and collecting system in patients with an upper posterior renal tumor.7,8 Fortunately, recently developed robotic partial nephrectomy is expected to solve several problems present in laparoscopic partial nephrectomy.
VAMS partial nephrectomy is a hybrid form of laparoscopic and conventional open surgery, combining the advantages of both. The unique characteristic of this technique is that it uses a surgical traction system, which provides optimum surgical space even with a small incision. Moreover, meticulous handling is possible through both three-dimensional direct vision via minilaparotomy and a simultaneously clearly magnified image on the monitor. This allows efficient and accurate dissection and suture techniques comparable to conventional and laparoscopic surgery. Dual decker system self-retaining retractors are used to maximize surgical space; therefore, the surgeon may perform surgery without assistance. Surgical instruments include modifications of conventional instruments used in open surgery and those used in laparoscopic surgery. VAMS for partial nephrectomy uses a retroperitoneal approach, which shortens the recovery period compared to open partial nephrectomy, and minimizes the risk of bowel injury compared to laparoscopic partial nephrectomy. However, the complication rate was similar across the three techniques.
Gasless renal surgery through small incisions is not new, and various renal surgeries are being performed in this manner for a variety of conditions, albeit with differences in equipment and technique.9,10 To our best knowledge, however, this study is the first direct comparison of video-assisted gasless minilaparotomy, open, and laparoscopic techniques in performing partial nephrectomy for renal masses in a large cohort of patients.
The present results showed that surgical, oncological, and renal functional outcomes are similar for VAMS, open, and laparoscopic partial nephrectomy. Furthemore, estimated blood loss, use of transfusion, complications, positive margins occurrence, disease-free survival, cancer-specific survival, and postoperative GFR were not significantly different among the three groups.
Partial nephrectomy is the standard treatment for the management of solitary kidney.11,12 We performed partial nephrectomy in 18 patients with tumors in a solitary kidney, by video-assisted technique in 12 patients, and by open technique in 6 patients. Findings of similar outcomes in these patients demonstrated that the VAMS technique can also be applied to solitary renal cell carcinomas in a nephron-sparing setting.
Many studies of partial nephrectomy report that positive margins occur in 1.0% to 7.4% of open procedures and 1.6% to 3.0% for laparoscopic procedures.13-17 Consistent with those reports, we found positive margins in 1.8% across the groups, without statistically significant difference among the groups. Many studies have demonstrated that open and laparoscopic access yield comparable oncologic outcomes. Moreover, disease-free survival and cancer-specific survival in our analysis, were similar among patients with renal cell carcinoma after VAMS, open, and laparoscopic partial nephrectomy.
Our VAMS partial nephrectomy technique has several merits that come from both open and laparoscopic surgical techniques. First, the VAMS approach uses the open technique of conventional surgery but has the advantage of being minimally invasive. Second, like the retroperitoneoscopic partial nephrectomy, which allows better access to posterior tumors, the VAMS approach is also extraperitoneal and prevents unnecessary bowel manipulation. Third, bleeding can be promptly controlled in case of a vascular accident. Fourth, conversion to open surgery is easy by simply extending the main incision, nevertheless, no conversion was necessary in the present series. Fifth, a minimal scar and early time to normal diet in patients after the VAMS approach are comparable to the laparoscopic approach.
The followings are limitations of this study. First, the data used in our study were collected retrospectively and reflects a single institution's experience. Second, data on the oncologic outcome must be interpreted with caution, because of rather short follow-up, especially shorter follow-up in the small number of patients in the laparoscopic group. Third, since VAMS, open, and laparoscopic partial nephrectomy were performed by different surgeons at our institution, the patients were not randomly assigned to the different techniques, and consequently, the outcomes may be influenced by the surgeons' experiences. However, we found that the surgical, oncological, and renal functional outcomes in patients after VAMS partial nephrectomy were similar to those reported previously. A larger sample size and longer follow-up are needed.
In conclusion, VAMS for partial nephrectomy is practiced in our institution as a minimally invasive technique that combines the advantages of laparoscopic and open partial nephrectomy. VAMS partial nephrectomy results in surgical, oncological, and functional outcomes similar to open and laparoscopic partial nephrectomy, which is the advantage of minimally invasive surgery. In addition, VAMS allows surgeons with no laparoscopic skills to approach laparoscopic recovery results in non-obese patients.
Figures and Tables
Fig. 1
(A) The self-retractor system for video-assisted minilaparotomy surgery for partial nephrectomy. (B) A laparoscopic retrieval sac (LapSac®, Sejong, Korea) was introduced through the minilaparotomy incision and placed around the kidney. (C) Laparoscopic bulldog clamps were applied to the renal artery. (D) The LapSac® was dissected in four parts, and ice slush was placed in the LapSac®. Tumor exposure was easily performed by handling these four parts of the LapSac®.
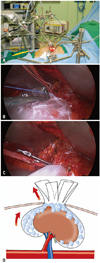
Fig. 2
Disease-free survival for 180 patients with renal cell carcinoma, stratified by VAMS, open, and laparoscopic techniques for partial nephrectomy (log rank test: VAMS vs. open, p=0.954; VAMS vs. laparoscopic, p=0.257; and open vs. laparoscopic, p=0.310). VAMS, video-assisted minilaparotomy surgery.

Table 1
Characteristics of 271 Patients Who Underwent Partial Nephrectomy, Based on Operative Method of Video-Assisted Minilaparotomy, Open, and Laparoscopic Techniques
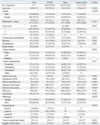
ASA, American Society of Anesthesiologists; BMI, body mass index; EBL, estimated blood loss; GFR, glomerular filtration rate; VAMS, video-assisted minilaparotomy surgery; RCC, renal cell carcinoma.
Data are presented as number (%) or mean±standard deviation.
GFR calculated as mL/min/1.73 m2.
*Statistically significant.
†Clavien-Dindo Classification.
ACKNOWLEDGEMENTS
Dong-Su Jang, Department of Anatomy, Yonsei University College of Medicine, assisted with photography.
References
1. Yang SC, Ko WJ, Byun YJ, Rha KH. Retroperitoneoscopy assisted live donor nephrectomy: the Yonsei experience. J Urol. 2001. 165:1099–1102.

2. Yang SC, Rha KH, Byun YJ, Kim WY. Video-assisted minilaparotomy in urology. J Endourol. 2003. 17:465–467.

3. Han WK, Lee HY, Jeon HG, Joo DJ, Rha KH, Yang SC. Quality of life comparison between open and retroperitoneal video-assisted minilaparotomy surgery for kidney donors. Transplant Proc. 2010. 42:1479–1483.

4. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999. 130:461–470.

5. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009. 250:187–196.
6. Jeong W, Park SY, Lorenzo EI, Oh CK, Han WK, Rha KH. Laparoscopic partial nephrectomy versus robot-assisted laparoscopic partial nephrectomy. J Endourol. 2009. 23:1457–1460.

7. Gill IS, Desai MM, Kaouk JH, Meraney AM, Murphy DP, Sung GT, et al. Laparoscopic partial nephrectomy for renal tumor: duplicating open surgical techniques. J Urol. 2002. 167:469–476.

8. Adkins KL, Chang SS, Cookson MS, Smith JA Jr. Partial nephrectomy safely preserves renal function in patients with a solitary kidney. J Urol. 2003. 169:79–81.

9. Suzuki K, Masuda H, Ushiyama T, Hata M, Fujita K, Kawabe K. Gasless laparoscopy-assisted nephrectomy without tissue morcellation for renal carcinoma. J Urol. 1995. 154:1685–1687.

10. Suzuki K, Ishikawa A, Ushiyama T, Fujita K. Retroperitoneoscopic living donor nephrectomy without gas insufflation: the five-year Hamamatsu University experience. Transplant Proc. 2002. 34:720–721.

11. Fergany AF, Saad IR, Woo L, Novick AC. Open partial nephrectomy for tumor in a solitary kidney: experience with 400 cases. J Urol. 2006. 175:1630–1633.

12. Gill IS, Colombo JR Jr, Moinzadeh A, Finelli A, Ukimura O, Tucker K, et al. Laparoscopic partial nephrectomy in solitary kidney. J Urol. 2006. 175:454–458.

13. Marszalek M, Meixl H, Polajnar M, Rauchenwald M, Jeschke K, Madersbacher S. Laparoscopic and open partial nephrectomy: a matched-pair comparison of 200 patients. Eur Urol. 2009. 55:1171–1178.

14. Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR Jr, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007. 178:41–46.

15. Kwon EO, Carver BS, Snyder ME, Russo P. Impact of positive surgical margins in patients undergoing partial nephrectomy for renal cortical tumours. BJU Int. 2007. 99:286–289.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download