Abstract
Immunoglobulin G4-related systemic disease (IgG4-RSD) is a recently defined emerging entity characterized by a diffuse or mass forming inflammatory reaction rich in IgG4-positive plasma cells associated with fibrosclerosis and obliterative phlebitis. IgG4-RSD usually affects middle aged and elderly patients, with a male predominance. It is associated with an elevated serum titer of IgG4, which acts as a marker for this recently characterized entity. The prototype is IgG4-related sclerosing pancreatitis or autoimmune pancreatitis (AIP). Other common sites of involvement are the hepatobiliary tract, salivary gland, orbit, and lymph node, however practically any organ can be involved, including upper aerodigestive tract, lung, aorta, mediastinum, retroperitoneum, soft tissue, skin, central nervous system, breast, kidney, and prostate. Fever or constitutional symptoms usually do not comprise part of the clinical picture. Laboratory findings detected include raised serum globulin, IgG and IgG4. An association with autoantibody detection (such as antinuclear antibodies and rheumatoid factor) is seen in some cases. Steroid therapy comprises the mainstay of treatment. Disease progression with involvement of multiple organ-sites may be encountered in a subset of cases and may follow a relapsing-remitting course. The principal histopathologic findings in several extranodal sites include lymphoplasmacytic infiltration, lymphoid follicle formation, sclerosis and obliterative phlebitis, along with atrophy and destruction of tissues. Immunohistochemical staining shows increased IgG4+ cells in the involved tissues (>50 per high-power field, with IgG4/IgG ratio >40%). IgG4-RSD may potentially be rarely associated with the development of lymphoma and carcinoma. However, the nature and pathogenesis of IgG4-RSD are yet to be fully elucidated and provide immense scope for further studies.
Immunoglobulin subtypes are significantly different in their underlying biologic regulation, distribution, and in their interaction with receptors on the various effector cells of the immune system. The immunoglobulin subtypes that are produced in response to a particular immune challenge depend on class-switch gene rearrangement of the constant regions.1 The immunoglobulin G (IgG) class is complex in structure and biology with four subclasses of IgG antibodies, ranging from 1 through 4 based upon the order of their discovery and serum levels. The serum concentration of IgG1 is the highest amongst the IgG subclasses and ranges from 5-11 mg/mL,2 whereas the least abundant subclass, IgG4, is present at mean concentrations of 0.35-0.51 mg/mL.3 The concentration of IgG4 in serum varies significantly among healthy people unlike the other IgG subclasses. IgG4 levels generally range from less than 10 µg/mL to 1.4 mg/mL, with levels over 2 mg/mL noted in rare instances and levels being generally higher in men and older people.2-4
IgG4 is a unique antibody based upon its structure, function, and immunologic regulation. IgG4 antibodies are distinct as they undergo 'half-antibody exchange' in vivo, resulting in recombined antibodies comprising two different binding specificities.5 IgG4 does not activate complement pathways and has reduced effector function relative to other IgG subtypes.6,7 IgG4 production is driven in part by T-helper cell 2 cytokines.8,9 IgG4 plays a significant role in bullous skin lesions,10 atopic eczema and bronchial asthma11,12 as well as decreasing IgE-mediated inflammatory response in parasitic infections.8,9 IgG4-related systemic disease (IgG4-RSD) represents a newly described category of diseases with a potential role for IgG4 antibodies. The exact role of IgG4 antibodies in the pathogenesis of IgG4-RSD still remains unclear in the present day scenario. An increased understanding of the precise role of IgG4 in these related clinical syndromes will be paramount for elucidation of the underlying pathophysiology.
Sarles, et al.13 raised the possibility of some cases of chronic pancreatitis arising from an autoimmune pathologic process in 1961. The concept of autoimmune pancreatitis (AIP) was proposed by Yoshida, et al.14 in 1995 along with the introduction of this terminology. Autoimmune pancreatitis has been recognized over the years as a distinct clinical entity, characterized by a mass lesion in the pancreas, painless obstructive jaundice, pancreatic duct narrowing, association with diabetes mellitus and favorable response to steroid therapy.
The autoimmune association of AIP is based upon its association with other immune-mediated diseases, such as sclerosing cholangitis, primary biliary cirrhosis (PBC), Sjogren syndrome, and inflammatory bowel disease (IBD).15,16 Autoantibodies, including antinuclear antibodies, rheumatoid factor, anticarbonic anhydrase II, and antilactoferrin are commonly encountered in patients with AIP.17-20 Kawa, et al.21 reported an association with HLA DRB1*0405-DQB1*0401 haplotype in a study from Japan. Numerous terminologies have been coined by different investigators to emphasize selected aspects of the disease, including chronic sclerosing pancreatitis,22 lymphoplasmacytic sclerosing pancreatitis,23 nonalcoholic duct-destructive chronic pancreatitis,24 idiopathic tumefactive chronic pancreatitis25 and duct-narrowing chronic pancreatitis.26 Over the past decade, developments have resulted in the establishment of the theory that AIP is a heterogeneous entity which includes at least 2 distinct clinicopathologic entities: 1) lymphoplasmacytic sclerosing pancreatitis (LPSP), and 2) idiopathic duct-centric chronic pancreatitis (IDCP).19,27,28
LPSP is a histologically unique lesion that was proposed by Kawaguchi, et al.23 in 1991. It consists of diffuse lymphoplasmacytic infiltration and fibrosis that focally gives rise to a storiform fibrosis. Neutrophils are conspicuously absent but eosinophils may be identified. Pancreatic lobules are relatively well preserved compared to alcoholic chronic pancreatitis, but focal destruction of pancreatic acini and replacement with fibrosis are routinely noted. This inflammatory process also characteristically extends around the main and interlobular ducts, leaving the duct epithelium and lumen intact. Veins are almost always obliterated by the same inflammatory process (obliterative phlebitis). Even the splenic and portal venous vasculature may be involved, leading to an intraoperative differential diagnosis of carcinoma. The common bile duct is usually inflamed resulting in jaundice seen in patients with AIP. Numerous IgG4-positive plasma cells are identified in LPSP on staining.29,30 LPSP predominantly affects elderly men with a mean age of 62 to 63.4 years, is commonly associated with proximal biliary tract, retroperitoneal, renal, and salivary disease, shows elevated serum IgG4 titer and relapse (47%) in a significant proportion of cases.31
IDCP is characterized by ductal epithelium centric inflammation.16,27,32 Neutrophilic infiltration in the main and/or interlobular ducts is typically seen, and extends to involve the duct epithelium and lumen. The duct epithelium shows destructive and regenerative changes with the lumen appearing stenotic or tortuous as a result of the inflammation. A band of lymphocytes and plasma cells surrounds the lumen but, in contrast to LPSP, the ductal lesion lacks the appearance of a thickened wall. The entire duct may seem entrapped within an aggregate of inflammatory cells. When the inflammation is severe, pancreatic lobules are also inflamed with neutrophils, lymphocytes and plasma cells and microabscesses may be seen as well. Although there is fibrosis around pancreatic lobules, inflammatory cells are scarce within fibrosis itself, in contrast to LPSP, in which inflammatory cells are numerous within fibrosis. Obliterative phlebitis is rare, and inflammation of the common bile duct is less common compared to LPSP. IgG4-positive plasma cells are usually few in IDCP.32
The clinical features of LPSP are concordant with those of AIP reported from Japan, described previously.27 Serum IgG4 is elevated in 80% of AIP patients in Japan, which correlates well with numerous IgG4-positive plasma cells seen in LPSP. In contrast, patients with IDCP are younger than LPSP patients, and many of them are younger than 40 years without any specific gender preponderance.27 Obstructive jaundice is less common in IDCP than in LPSP. The association of IBD is found in IDCP, but extrapancreatic manifestations seen in LPSP are rare. It is noteworthy that IDCP is rare in Japan.33
Owing to the fact that both LPSP and IDCP have overlapping clinicopathological features, there has been a contentious debate as to whether these two pathological groups are different manifestations of a single entity of AIP, or whether they are distinct clinicopathological entities. The controversy is due to the variety of AIP diagnostic criteria proposed by different groups. The diagnostic criteria from Japan,34 Korea,35 consensus of the Japan-Korea symposium36 and Mayo Clinic37 define LPSP as the pathological entity of AIP, but other groups include both LPSP and IDCP in AIP.16,32,38 The theory that LPSP and IDCP are distinct entities has gained acceptance due to the differing demographic and clinical presentations as well as different immunoreactivity for IgG4. Recently, new nomenclature, type 1 and type 2 AIP, which correspond to LPSP and IDCP, respectively, have been proposed.39
In 2001, Hamano, et al.40 reported elevated serum IgG4 levels in a cohort of patients with a specific subtype of sclerosing pancreatitis/AIP. This finding was not observed in patients with pancreatic carcinoma, primary sclerosing cholangitis (PSC), PBC, nonspecific chronic pancreatitis, Sjogren syndrome and normal subjects. Kamisawa, et al.41 reported that IgG4-positive plasma cells are increased systemically in patients with AIP and concluded that AIP patients have a systemic disease, with the proposal for the entity 'IgG4-related sclerosing disease'.42 Histologically, this subtype corresponds to LPSP, but not IDCP.30,32,43
IgG4-related sclerosing pancreatitis is seen most commonly in middle-aged and elderly men with a mean age of 59 to 68 years and a male-to-female ratio of 4-7.5: 1.44-46 The prevalence rate is 2% to 11% among patients with chronic pancreatitis.20,45 Studies have demonstrated that it accounts for up to one third of the total cases of benign conditions that are treated with pancreatoduodenectomies, thus highlighting the fact that is often mistaken for pancreatic cancer in spite of major advancements in clinical medicine and radiology.47,48
Patients usually present with a pancreatic mass and/or painless obstructive jaundice. Newly diagnosed type II diabetes mellitus and steatorrhea are noted in some of these cases.20,37 Contrarily, systemic symptoms, including fever, weight loss, and generalized malaise are rarely seen. Imaging studies characteristically show enlargement of the pancreas, predominantly involving the pancreatic head, and endoscopic retrograde cholangiopancreatography (ERCP) shows irregular narrowing of the pancreatic duct with or without stenosis of the common bile duct.36
The disease responds well to steroid therapy, although relapses can occur on cessation of treatment.37 Response to treatment is monitored by a decline in serum IgG4 titer and a reduction in IgG4+ cells in the affected organs. Rituximab administration may be significantly beneficial according to a recent study which reports an excellent response in IgG4-related pancreatitis cases.49 The drug acts by reducing the number of B-lymphocytes that provide a source for the IgG4-secreting plasma cells.
The cut surface of the pancreas is firm with tan-white areas interspersed amidst the lobulated yellow-gray parenchyma (Fig. 1). The histologic triad necessary for a diagnosis comprises of the following: 1) lymphoplasmacytic infiltrate, 2) sclerosis, and 3) obliterative phlebitis, with accompanying atrophy and destruction of pancreatic acini although these morphologic features are characteristic, they are not completely specific, and thus a definitive diagnosis requires the demonstration of an increased population of IgG4+ plasma cells.31
Sclerosis of the interlobular septa further enhances the lobular architecture of the pancreas by making the separate lobules appear more demarcated (Fig. 2A). The extent of involvement varies greatly from one lobule to another. This may range from normal appearing lobules, to moderate atrophy and inflammation, and complete replacement by a fibroinflammatory process. The pancreatic lobules show interstitial infiltration by plasma cells, reactive lymphocytes, and a variable number of eosinophils, accompanied by atrophy or loss of acini (Fig. 2B). The lobular stroma shows variable degrees of sclerosis, which can exhibit a storiform character.36,37 Prominent edema or myxoid change may be noted. Increasing degrees of sclerosis results in loss of the lobular architecture.50 The fibrosclerosis and inflammatory process can extend beyond the confines of the pancreas into the retroperitoneal tissue and surrounding organs. Islet cell hyperplasia is a rare finding in contradistinction to the routinely encountered examples of chronic pancreatitis. The pancreatic ducts appear more prominent as a result of being surrounded by a periductal cuff of chronic inflammatory cells, accompanied by a sclerosing stromal reaction. The ductal epithelium is not infiltrated by inflammatory cells. Irregular narrowing of the ductal lumen gives rise to the characteristic imaging findings.
Phlebitis, characterized by transmural inflammatory infiltration of the veins, with or without fibrous obliteration of the lumen, is a sine qua non of this disease (Fig. 2C).31 An elastic stain may highlight the venous obliteration. Lymphoid follicles can be present in the stroma. Sometimes fibroinflammatory mass lesions are formed, which have been called "inflammatory pseudotumor" in the literature.51 Another noteworthy feature often not reported is the tendency for the inflammatory cells to form cuffs around nerves. Plasma cells and lymphocytes can even infiltrate into the nerves.31
A preoperative diagnosis of IgG4-related sclerosing pancreatitis is necessitated by the need to avoid surgery in these cases which are otherwise treated with steroids.52 Although there is no definite cut-off limit for the number of IgG4+ plasma cells currently, greater than 30 IgG4+ plasma cells per high-power field has been reported as being specific.30,32 A recent study suggests a cut-off level of more than 50 per high-power field IgG4-positive cells to be more specific (Fig. 2D).53 In nonspecific chronic inflammation or pancreatic carcinoma, few scattered IgG4+ cells are seen. The number of IgG4+ plasma cells is more sensitive and specific than serum IgG4 titer as the latter is not elevated in approximately one third of cases but may be elevated in other conditions such as in patients with pancreatic carcinoma.54-56 Alternatively, the ratio of IgG4+/IgG+ cells (>40%) can be used as a standard parameter to arrive at a sensitive and specific diagnosis.43,57,58
The diagnosis of IgG4-related sclerosing pancreatitis is usually rendered upon consideration of clinical, laboratory, imaging, and histologic features. Biopsies may be falsely negative since the pancreas may not be involved in its entirety.59 ERCP guided biopsies of the pancreas may be more helpful in demonstrating the presence of IgG4+ plasma cells.60 The diagnostic criteria have undergone several changes and vary from one continent to another (Table 1 and 2). However, the diagnosis is almost always determined by the discovery of increased number of IgG4-positive plasma cells coupled with the morphologic findings. The diagnosis can be rendered possible in the absence of biopsy tissue with the aid of imaging studies, extrapancreatic organ involvement, and response to steroids may help in establishing the diagnosis if tissue samples are not available.36,37,61
IgG4-related sclerosing pancreatitis is frequently associated with extrapancreatic lesions, and the spectrum of disease manifestations involving different organs has continued to expand.20,37,61-72 Hamano, et al.62 reported that extrapancreatic bile duct lesions occur in 73.9%, hilar lymphadenopathy occurs in 80.4%, lacrimal and salivary gland lesions in 39.1%, hypothyroidism in 22.2%, and retroperitoneal fibrosis in 12.5% of cases with IgG4-RSD. The various sites of involvement exhibit similar morphologic features with the common denominator being a significant increase in the number of IgG4+ plasma cells (Table 3).29,41,42
Extrapancreatic involvement may develop prior to, concomitantly or following pancreatic disease, or may even occur independently in the absence of pancreatitis. A so called association of "autoimmune pancreatitis" with other "autoimmune diseases" including PSC, Sjogren syndrome, and retroperitoneal fibrosis simply represents extrapancreatic manifestations of a single systemic entity rather than a multitude of diseases.39,73,74 Another pointer favoring this theory states that an increased number of IgG4+ plasma cells can be identified in other uninvolved organs in such cases organs, such as the aerodigestive tract mucosa and bone marrow, thus providing credence in favor of the systemic nature of this disease.42,65,75,76 The various names ascribed to this disease include IgG4-related sclerosing disease,77 IgG4-related systemic sclerosing disease,78 IgG4-related disease,61,79 IgG4-related autoimmune disease,42 hyper-IgG4 disease,80 and IgG4-related systemic disease.49
The extrapancreatic lesions of IgG4-related sclerosing disease also exhibit the same characteristic histologic features including lymphoplasmacytic infiltrate, sclerosis, and obliterative phlebitis as similarly seen in IgG4-related sclerosing pancreatitis. The relative proportion of the lymphoplasmacytic infiltrate and sclerosis in different cases gives rise to a spectrum of histologic patterns: pseudolymphomatous, mixed, and sclerosing. These patterns represent different stages in the evolution of this disease with the sequence progressing from being initially pseudolymphomatous to mixed and subsequent progression to the sclerosing pattern. The pseudolymphomatous pattern is more commonly identified in superficial locations including lacrimal gland, salivary gland, breast, and skin and thus these are detected earlier than deep seated sclerotic lesions in locations such as the retroperitoneum.31
The pseudolymphomatous pattern is characterized by a mass forming dense infiltrate of small lymphocytes and plasma cells, with interspersed reactive lymphoid follicles and a variable proportion of scattered eosinophils. The differential diagnosis includes low grade non-Hodgkin's lymphoma, particularly extranodal marginal zone lymphoma. However, these can be distinguished based on the absence of lymphoepithelial lesions, monocytoid B-cells, diffuse sheets of CD20+ B cells, light chain restriction, aberrant immunophenotype, and clonal immunoglobulin gene rearrangements.31
The mixed pattern is the most common, comprising of patchy dense infiltrates of small lymphocytes and plasma cells that are accompanied by significant sclerosis (Fig. 2B). Variable numbers of eosinophils and reactive lymphoid follicles are usually seen. The sclerosing pattern is characterized by a predominantly sclerotic process with poorly defined borders and irregular extensions into the surrounding tissues. Patchy aggregates of lymphocytes and plasma cells, with or without lymphoid follicle formation, are seen diffusely scattered over the extent of the lesion, and are seen more commonly at the periphery than at the center of the lesion. The sclerotic areas comprise of collagen deposition with scattered fibroblasts. Obliterative phlebitis is often seen except in small biopsies.31 The main differential diagnoses are nonspecific inflammation with fibrosis, and malignancies with sclerosis including nodular sclerosing Hodgkin lymphoma, sclerosing large B-cell lymphoma, primary or metastatic carcinoma with desmoplastic stromal changes. The veins show segmental or circumferential transmural infiltration by chronic inflammatory cells, and can show luminal occlusion by fibrous tissue.31 Obliterated veins can be difficult to recognize and may necessitate the aid of elastin stains for identification purposes.
Biliary tract involvement (IgG4-related sclerosing cholangitis) is seen in 50% to 90% of patients with IgG4-related sclerosing pancreatitis and clinically presents as obstructive jaundice or fever.23,51,81-84 Biliary tract disease may also be seen in some cases without pancreatic involvement. Both the extrahepatic and intrahepatic bile duct systems can be affected with the prior being predominantly involved in most reported cases.85 As seen with AIP, the differential diagnosis includes a malignancy of the bile duct system.81-85 The involved bile ducts demonstrate a transmural lymphoplasmacytic infiltrate with variable numbers of eosinophils, sclerosis (Fig. 3A), and obliterative phlebitis (Fig. 3B). The bile duct lumina undergo stenosis owing to the periductal fibrosis and inflammation (Fig. 3C) and the peribiliary glands are often involved in this region.85 Immunohistochemical staining for IgG4 demonstrates a significant subset as being IgG4-positive plasma cells (Fig. 3D).
The clinical and pathologic features overlap with those seen in PSC, a chronic biliary inflammatory disease of unknown etiology. The pathologic process in PSC also involves prominent ductal and periductal fibrosis, luminal stenosis, obliteration, and dilatation.86 It is thought that some of the cases reported as PSC actually represent IgG4-related sclerosing cholangitis.87-91 Mendes, et al.92 published a series of cases of PSC wherein elevated serum IgG4 was found in 9% of the patients and this group of patients also had a lower frequency of IBD.
In IgG4-RSD involving the bile ducts, the bile duct epithelium is usually spared but the peribiliary glands exhibit a greater degree of involvement and inflammation tends to be more severe toward the adventitial aspect. Onion-skin type concentric periductal fibrosis, which is a diagnostic hallmark of PSC, is not seen in IgG4-related cholangitis.81-84 Another distinguishing feature is that a large number of IgG4-positive plasma cells are identified in IgG4-related cholangitis but not in PSC. PSC is a progressive disease culminating in cirrhosis with liver failure requiring transplantation, whereas IgG4-related sclerosing cholangitis is treated with steroid therapy in most cases.81-84,93
The gall bladder may be involved in up to one fourth of patients with IgG4-related sclerosing pancreatitis with the most common manifestation being acute acalculous cholecystitis.94 Co-existing IgG4-related sclerosing cholangitis is also frequently encountered. IgG4-related cholecystitis shows predominant extramural involvement with adventitial inflammation and subserosal inflammatory nodules in contrast to the nonspecific acalculous cholecystitis, which is seen occasionally in patients with PSC, pancreatic cancer, and biliary calculi.58,95
Hepatic involvement is also frequently identified with IgG4-related cholangitis or pancreatitis. The commonest presentation is seen as a mass lesion often referred to as an "inflammatory pseudotumor".51,96,97 The lesion usually involves the hilum of the liver and is bile duct centric with the usual diagnostic triad of a lymphoplasmacytic infiltrate, sclerosis, and obliterative phlebitis. IgG4-related hepatic "inflammatory pseudotumor" might represent an extension of IgG4-related sclerosing cholangitis into surrounding hepatic tissue.31,51,96,97
This IgG4-related lesion is morphologically different from soft tissue related lesions including inflammatory pseudotumor/inflammatory myofibroblastic tumor of the liver since it does not comprise of a spindle cell or myofibroblastic proliferation, does not express the ALK protein frequently associated with inflammatory myofibroblastic tumor and instead comprises of numerous IgG4+ plasma cells with surrounding sclerosis and phlebitis.98 Follicular dendritic cell sarcoma is another entity which must be included in the differential diagnosis of these lesions but can be distinguished by the presence of intersecting fascicles of spindle cells as well as positivity for follicular dendritic cell markers (CD21 and CD35), and Epstein-Barr virus in situ hybridization studies.31,99
Different morphologic patterns are appreciated in liver biopsies obtained from patients with IgG4-RSD. These include portal inflammation with or without interface hepatitis, portal sclerosis, large bile-duct obstructive features, lobular hepatitis, and canalicular cholestasis.100 The number of IgG4+ plasma cells is concomitantly increased and helps to distinguish IgG4-related hepatic disease from other differentials including PSC, autoimmune hepatitis, primary biliary sclerosis, and chronic viral hepatitis in which only far fewer IgG4+ plasma cells are identified.100,101
The submandibular gland is the salivary gland most frequently involved by IgG4-RSD and is also referred to as 'chronic sclerosing sialadenitis' or 'Kuttner tumor'. The disease was originally described by H. Kuttner102 in 1896, who reported it as a unilateral or bilateral "hard swelling" of the submandibular glands. Chronic sclerosing sialadenitis demonstrates a predilection for the submandibular gland, although parotid gland involvement has also been reported.103 It is characterized by the presence of a prominent lymphoplasmacytic infiltrate and lymphoid follicles, which upon being combined with the finding of cytotoxic T cell populations, indicate an immunological process.104
Clinical manifestations include unilateral or bilateral hard masses that are often mistaken for a tumor based on both clinical and radiological features.57,61,64,105-107 Almost all cases of chronic sclerosing sialadenitis have been shown to be IgG4-related in the submandibular gland, whereas up to a third of all cases of nonspecific chronic sialadenitis are IgG4-related.57,61,64 Recognition of IgG4-RSD in the salivary glands is significantly important as these patients may develop IgG4-related lesions in other body sites as well. The parotid glands can be involved either solely or along with the submandibular glands. The morphologic features of salivary gland involvement are identical to those observed in other organs.57,61,64 It is characterized by sclerosis of interlobular septae with resulting accentuation of the salivary gland lobular architecture and an interstitial infiltrate of lymphocytes and plasma cells along with variable degrees of sclerosis. The involvement of adjacent salivary lobules may be non-uniform with focal atrophy and loss of acini in some lobules. Reactive lymphoid follicles and collagenous sheaths around small ducts are frequently seen.57,61,64
IgG4-related sclerosing dacryoadenitis and/or sialadenitis is one of the major causes of Mikulicz disease with bilateral lacrimal and salivary glands involvement.108-110 Mickulicz disease, which can represent a number of clinical etiologic entities, is defined clinically as bilateral, painless, and symmetrical swelling of the lacrimal, parotid, and submandibular glands of unknown etiology for a duration exceeding 3 months. It is not synonymous with the entity Sjogren syndrome, a distinct autoimmune disease featuring keratoconjunctivitis sicca, xerostomia, intermittent swelling of the salivary or lacrimal glands, presence of anti-Ro/SSA and anti-La/SSB autoantibodies, and morphologically characterized by lymphoepithelial sialadenitis.110
IgG4-RSD commonly affects the lacrimal glands and surrounding orbital soft tissues and these were the initial sites reported to be involved by this disease apart from the pancreas. The patients usually present with unilateral or bilateral, painless orbital swellings of long duration with or without significant impairment of visual acuity or symptoms of 'dry eye'.60,65 The differential diagnosis on clinical and radiological examination includes inflammatory pseudotumor and lymphoma. Extensive involvement of orbital soft tissues and sclerosis may lead to markedly decreased visual acuity and occasionally cause optic nerve atrophy and blindness.65 Steroid therapy is the primary treatment but may fail to ameliorate the condition in severe cases with irreversible sclerosis and fibrosis of orbital tissues.60,65
The morphologic features of lacrimal gland involvement (IgG4-related chronic sclerosing dacryoadenitis) are similar to those of IgG4-related sclerosing disease in other organs, except that obliterative phlebitis is not as commonly seen.60,65 Progressive sclerosis and gland destruction frequently comprise the course of this disease in the orbit with an eventual burnout phase where only the fibrosis remains in the absence of increased inflammation. It may cause extensive fibrosis to the degree that no recognizable lacrimal gland or orbital structure is identified on biopsies. All patterns of inflammation and fibrosis, including pseudolymphomatous, mixed and sclerotic patterns, are identified in the lacrimal gland and orbital tissues.31,60,65
Inflammatory fibrosclerosing lesion involving the retroperitoneum, mesentery and mediastinum is an idiopathic entity characterized by diffuse fibrosis and is confused with inflammatory pseudotumor or inflammatory myofibroblastic tumor.111 Idiopathic retroperitoneal fibrosis or mediastinal fibrosis may be associated with Riedel thyroiditis, orbital/lacrimal IgG4-RSD or IgG4-related sclerosing cholangitis.112 The fibrosis in these locations may extend to involve the kidney, ureter and bowel mesentery with varying response to steroid therapy which is ineffective in advanced fibrosis.113-118
Noninfectious aortitis is represented by a group of inflammatory disorders characterized by chronic inflammation within the aortic wall, wherein the evident inflammation is not considered to be due to infection.119 Noninfectious aortitis is most commonly seen involving the thoracic aorta, especially the ascending aorta, and may result from well known systemic rheumatologic disorders such as giant cell arteritis, Takayasu arteritis, rheumatoid arthritis, ankylosing spondylitis, and Behcet's disease.120,121
The lesions described above are often characterized by a variably prominent lymphoplasmacytic infiltrate, sclerosis, and phlebitis which are essentially identical to those of IgG4-RSD.122 A frequent association with pancreatitis has also been reported in several cases.118,123 Upon extrapolating these findings, a significant proportion of cases of retroperitoneal fibrosis,124,125 mediastinal fibrosis,67 sclerosing mesenteritis,126 periaortitis and inflammatory aortic aneurysm (with cases predominantly involving abdominal aorta with rare cases involving aortic arch)71,127-132 have been demonstrated to express markedly increased numbers of IgG4+ plasma cells, and hence represent members of IgG4-RSD. It has not been determined at present whether the group with no significant increase in IgG4+ cells has different etiologies or represents burnt out IgG4-RSD.31
Involvement of the thyroid gland as part of the spectrum of IgG4-RSD is being reported increasingly. Patients with AIP often have concomitant evidence of hypothyroidism, with increased levels of antithyroglobulin antibody.133 The exact pathogenetic mechanism for this phenomenon has yet to be elucidated. A study by Li, et al.134,135 involved segregating 70 cases of Hashimoto thyroiditis into 2 groups: 19 IgG4-related cases and 51 non-IgG4-related cases. The IgG4-related group comprised of cases with a greater degree of stromal fibrosis, lymphoplasmacytic infiltration, and thyroid follicular cell destruction than the other group. The IgG4-related subset is associated with a higher level of circulating thyroid autoantibodies, lower female-to-male ratio and an aggressive clinical course. It is possible that hypothyroidism in patients with autoimmune pancreatitis may result from an IgG4-related thyroiditis which may be mistaken for Hashimoto thyroiditis in view of the overlapping symptoms and laboratory findings. Riedel thyroiditis is also regarded as a component of the multifocal fibrosclerosis seen in IgG4-RSD with reports backing this theory based on immunohistochemical findings.136-138
Lung involvement by IgG4-RSD may present insidiously with the usual pulmonary symptoms including cough, chest pain, dyspnea and hemoptysis.69,139-141 Radiologically, it may appear as solitary or multiple lung nodules, or consolidation and hilar lymphadenopathy.142 Distinct patterns of pulmonary involvement include: solid nodules, peribronchial or perivascular, and interstitial alveolar infiltrates.140
The solid nodular type comprises a hilar or peripheral mass forming lesion with the characteristic lymphoplasmacytic infiltrate and accompanying sclerosis.69 The adjacent alveoli show interstitial infiltration of lymphocytes and plasma cells. In cases involving the hilum, sclerosing inflammation of the bronchial wall is identified and it is almost always accompanied by involvement of the bronchial glands. The bronchovascular type is characterized by lymphoplasmacytic infiltrate distributed along the lymphatic channels, with interstitial expansion and extension along the bronchovascular bundles, interlobular septa, and pleura. Lymphatic dilatation with histiocytes showing emperipolesis of lymphocytes is a helpful identifying feature.139 In the alveolar interstitial type, the lymphoplasmacytic infiltrate is limited to the alveolar interstitium and is difficult to distinguish from nonspecific interstitial pneumonia.
A variable population of eosinophils along with lymphoid follicles is routinely seen in up to 50% of these cases. One distinguishing feature noted in pulmonary cases of IgG4-RSD is that the arteries are also frequently involved and obliterated as opposed to other body sites wherein veins are selectively involved.139,140 Endothelialitis characterized by a lymphoplasmacytic infiltrate in the subendothelium leading to narrowing and obliteration of the vascular lumen is often identified. Visceral or parietal pleural involvement is noted usually in the form of fibrous thickening accompanied by a chronic lymphoplasmacytic infiltrate with or without fibrinous exudates.139-141
IgG4-related sclerosing mastitis can present as single or multiple unilateral or bilateral painless masses, with or without evidence of systemic IgG4-RSD.70,143 Morphologically, it is characterized by a prominent lymphoplasmacytic infiltrates with lymphoid follicle formation, patchy fibrosis, and atrophy of breast lobules. Phlebitis is also seen in some cases. The differential diagnosis includes involvement of the breast by lymphoma owing to the dense lymphoplasmacytic infiltrate or hyaline-vascular variant of Castleman disease if follicles with regressive changes are seen.31
IgG4-RSD may also involve the kidneys and urinary tract leading to disturbances in renal function.144-147 Radiologic studies may demonstrate the presence of poorly circumscribed nodular masses in the kidneys, often necessitating the exclusion of malignancy.145 The commonest findings on biopsy or nephrectomy are tubulointerstitial nephritis with an IgG4-positive plasma cell-rich inflammatory infiltrate, fibrosis, and tubular atrophy. IgG4 electron-dense deposits have been demonstrated in the peritubular basement membrane using electron microscopy. The renal glomeruli are usually uninvolved but few cases with concurrent membranous nephropathy have been recorded.144-148 The tubulointerstitial nephritis is steroid responsive, whereas the response of membranous glomerulonephritis can be variable. IgG4-related chronic sclerosing pyelitis and IgG4-associated inflammatory pseudotumor of the ureter presenting as hydronephrosis have also been reported (Fig. 4).149,150
Central nervous system involvement by IgG4-RSD is extremely rare, and the most commonly reported site is the pituitary gland (IgG4-related infundibulo-hypophysitis). A male predominance is noted. The clinical picture includes patients presenting with hypopituitarism, diabetes insipidus, and/or symptomatic local mass effect.151-156
A pituitary mass and/or thickening of the pituitary stalk are identified on imaging studies. The treatment modality is steroid therapy which usually elicits a favorable response and manifests as a marked reduction in lesional size and correction of hormonal imbalances.151-156 An accompanying meningeal involvement in the form of hypertrophic pachymeningitis or sinus involvement, with extension to the sellar and parasellar regions is identified in some cases.152 The pituitary shows patchy or extensive interstitial infiltration of lymphocytes and plasma cells, along with loss of pituitary parenchyma.
Rare cases of dural spinal involvement have been reported (IgG4-related sclerosing pachymeningitis).157 Such lesions described as idiopathic hypertrophic pachymeningitis may represent part of the spectrum of IgG4-RSD.158,159 It is still unclear whether a few reported cases of intracranial inflammatory pseudotumor with increased IgG4+ plasma cells reported by Lui, et al.160 actually represent IgG4-RSD since these patients did not exhibit any other manifestations of systemic IgG4-RSD.
Skin involvement in IgG4-RSD may be either isolated or as part of systemic IgG4-RSD.68,161 The clinical presentation is in the form of cutaneous plaques or nodules tending to involve the head and neck. Microscopic examination reveals a dermal and subcutaneous nodular lymphoplasmacytic infiltrate with formation of lymphoid follicles and dermal or subcutaneous sclerosis. A markedly increased number of IgG4+ plasma cells, small lymphocytes, and variable infiltrate of eosinophils is identified.31
Cases of IgG4-RSD have been reported in the pericardium,162 prostate,163 seminal vesicles,164 nose, paranasal sinuses165,166 and uterine cervix (idiopathic cervical fibrosis).167 Questionable entities currently include autoimmune esophagitis and splenic sclerosing angiomatoid nodular transformation, which exhibit some of the features overlapping with IgG4-RSD, including increased plasma cells and sclerosis.168-171 However, there is no definite evidence to currently include these lesions under the spectrum of IgG4-RSD.
Lymphadenopathy is identified in up to 80% of patients with autoimmune (IgG4-related sclerosing) pancreatitis as evidenced by imaging studies.62 Lymph node involvement may be multifocal with the commonest nodes involved being mediastinal, abdominal, and axillary lymph nodes. Clinical manifestations vary greatly.66,161,172 Enlarged asymptomatic lymph nodes are identified in surgical resection specimens or identified on imaging studies of patients with known IgG4-RSD. Lymphadenopathy may also develop following an established diagnosis of extranodal IgG4-RSD or may be the initial presenting manifestation with subsequent discovery of systemic IgG4-RSD. The enlarged lymph nodes may be asymptomatic or may give rise to a mass effect in various body sites.
The differential diagnosis in these cases with lymphadenopathy includes lymphoma, multicentric Castleman disease, or metastatic malignant tumor. The lymph nodes are usually not exceedingly enlarged (≤2 cm) and not associated with fever and weight loss. Serum lactate dehydrogenase level is normal or only slightly elevated. Other laboratory findings include raised serum IgG4, serum IgG, polyclonal hypergammaglobulinemia and elevated erythrocyte sedimentation rate (ESR).66,161
The morphologic features of IgG4-related lymphadenopathy are different from those of extranodal sites since there is usually no sclerosis or phlebitis. Five histologic patterns of IgG4-related lymphadenopathy have been described.66,79,161 The various subtypes are as follows: multicentric Castleman disease-like pattern (type I),172-178 follicular hyperplasia pattern (type II),66 interfollicular plasmacytosis and immunoblastosis pattern (type III),66,79,161 progressive transformation of germinal center-like pattern (type IV),79,179 and nodal inflammatory pseudotumor-like pattern (type V).79,179,180 Abundant IgG4+ polyclonal plasma cells are present in interfollicular and follicle center compartments in all of these patterns (Fig. 5).79
Sato, et al.79 have reported cases of ocular marginal zone lymphoma associated with ocular adnexal IgG4-related sclerosing disease. A few cases of localized non-Hodgkin's lymphoma (marginal zone lymphoma and follicular lymphoma) arising in a background of IgG4-related chronic sclerosing dacryoadenitis have been recently reported.181 A rare example of composite marginal zone lymphoma and classical Hodgkin lymphoma associated with IgG4-related sclerosing disease of the cervical soft tissue has also been published.167 A recent study from the Mayo Clinic cites 3 cases of lymphoma (2 large B-cell lymphomas and 1 B-cell lymphoma unspecified) discovered on follow-up of 111 patients with IgG4-related sclerosing disease, suggesting that the syndrome increases the risk of developing lymphoma in various body sites.182
Several cases of pancreatic ductal adenocarcinoma have been published in association with IgG4-RSD.183-186 Case reports of pulmonary adenocarcinoma, salivary duct carcinoma, urothelial carcinoma in situ and gastrointestinal clear cell sarcoma in association with IgG4-RSD have been reported in the lung, parotid gland, ureter and small intestine, respectively.140,150,187,188 Further studies are mandated in this field to determine if there is any causal relationship between the malignancies and IgG4-RSD.
The pathogenesis of IgG4-related sclerosing disease is still not clearly understood. The disease may represent a hypersensitive/allergic reaction as compared to being an autoimmune disease.189 An association of IgG4-related AIP with gastric ulcer and Helicobacter pylori (H. pylori) infection has been proposed.190-192 It has been theorized by Okazaki, et al.193 that the development of the acronym 'AIP' involves a biphasic mechanism in which the initial response to self-antigens such as carbonic anhydrase, lactoferrin, pancreatic secretory trypsin inhibitor and ά-fodrin and molecular mimicry (H. pylori) are induced by decreased naive regulatory T cells, in conjunction with T-helper 1 cells releasing proinflammatory cytokines. Progression is mediated through increased memory regulatory T cell and T-helper 2 cell immune responses resulting in chronic inflammation. The inflammatory infiltrate usually comprises of a mixed population of T and B cells, with increased CD4+, CD25+, FOXP3+ regulatory T cells.31,194,195 T-helper cell 2 cytokines (interleukin-4, interleukin-5, and interleukin-13) and regulatory cytokines (interleukin-10 and transforming growth factor) are upregulated in the involved tissues.194-196 Other proposed hypothesis regarding the pathogenesis include enhanced T-helper type 2 responses to intestinal microflora197 and an immune-mediated pathogenesis based on the ultrastructural finding of electron dense immune complex deposits along the basement membranes of pancreatic acini and renal tubules.32
According to the results of a recent study conducted by screening of pooled IgG from patients with AIP, a specific reactivity with a peptide demonstrating homology with plasminogen-binding protein of H. pylori and with ubiquitin-protein ligase E3 component n-recognin 2 (an enzyme with high levels of expression in pancreatic acinar cells) was noted in the serum samples from 90% of patients with AIP and 10% of patients with pancreatic cancer. Healthy controls did not show any expression.198 It remains to be seen whether H. pylori truly plays a role in the development of AIP and IgG4-RSD.
Diagnosis of IgG4-RSD requires not only an increase in the absolute number of IgG4+ cells but also an increased IgG4+/IgG+ ratio for the correct diagnosis. An absolute IgG4+ plasma cell count is not sufficient as a sole index as IgG4+ cells normally comprise approximately 5% of all IgG+ cells, and may be present in significant numbers in any inflammatory lesions with increased plasma cells even though the percentage might remain in the acceptable range. A high IgG4+/IgG+ percentage alone is also not sufficient for a diagnosis because in cases with a few plasma cells, an erroneously high ratio may be calculated owing to the relative proportions of both IgG and IgG4+ plasma cells. We are in agreement with other investigators with regard to the cut-offs for the absolute number of IgG4+ cells >50/high-power fields (HPF) and ratio of IgG4+/IgG+ cells >40%.31 We also propose selecting areas with the maximum density of IgG4+ plasma cells. At least three HPFs should be counted, and the average number of IgG4+ plasma cells per HPF should be calculated to give the results. In borderline cases, a descriptive diagnosis with a comment indicating "increased IgG4+ plasma cells" should be rendered along with a disclaimer for the clinician requesting additional work-up or follow-up to determine if the lesion indeed represents IgG4-RSD.31 A raised serum IgG4 level is not mandatory for the diagnosis but may be of valuable assistance. Serum IgG4 titer often correlates with disease activity and the number of involved organs, but levels may also be normal.40 It is noteworthy that increased serum IgG4 has also been reported in a host of other diseases, including atopic dermatitis, parasitic infections, pemphigus vulgaris, pemphigus foliaceus, and pancreatic carcinoma.
Not every entity with increased IgG4+ plasma cells and a high IgG4/IgG ratio can be accepted as belonging to the spectrum of IgG4-RSD. Requirements such as concomitant morphologic features and an appropriate clinical context are mandatory for diagnosis. Autoimmune diseases include cases with an increased proportion of IgG4+ plasma cells but this does not qualify for inclusion as IgG4-RSD because the morphologic features and clinical findings do not correspond with the diagnostic criteria.
In the past decade, significant progress has been made in our understanding of the genetic and immunologic triggers contributing to IgG4-RSD in addition to the discovery of the vast clinicopathologic spectrum of this entity. Our knowledge is currently evolving as newer developments come to light revealing a widely expanding histopathologic spectrum in this disease entity. Further studies are necessary to delineate the precise role played by IgG4 in the pathogenesis of this disease group in order to make meaningful contributions in the management of patients with this disease.
Figures and Tables
Fig. 1
The gross photograph of pancreas shows an ill-defined firm lesion in the head of the pancreas (arrow) with yellow-tan and fibrotic cut surface. The lesion extends into the peripancreatic fat tissue.

Fig. 2
Microscopic features of IgG4-related sclerosing pancreatitis. (A) Pancreatic lobules are replaced by severe inflammatory cell infiltration and fibrosis. Sclerosis of interlobular septa enhances lobular architecture of pancreas (H&E stain, original magnification ×40). (B) The lobular interstitium is infiltrated by plasma cells, lymphocytes, and a variable number of eosinophils. Residual atrophic pancreatic acini are identified (right upper corner, arrow; H&E stain, original magnification ×400). (C) Obliterative phlebitis is characterized by perivenular and intravenular inflammatory cell infiltrate with fibrous obliteration of the lumen (H&E stain, original magnification ×200). (D) IgG4 immunostain shows markedly increased IgG4-positive plasma cells (>50 plasma cells/HPF; immunohistochemical stain, original magnification ×200). IgG4, immunoglobulin G4; HPF, high-power fields.
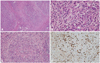
Fig. 3
Microscopic features of IgG4-related sclerosing cholangitis. (A) Intrahepatic bile ducts are infiltrated by lymphoplasmacytes with variable numbers of eosinophils, intermixed with collagen fibers (H&E stain, original magnification ×400). (B) Lymphoplasmacytes are infiltrating in the perivenular soft tissue of large veins, resulting in vascular wall destruction and luminal obliteration (H&E stain, original magnification ×200). (C) The bile duct lumen undergoes stenosis due to the periductal fibrosis and inflammation (H&E stain, original magnification ×40). (D) Numerous plasma cells are positive for IgG4 (immunohistochemical stain, original magnification ×200). IgG4, immunoglobulin G4.
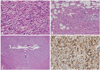
Fig. 4
Microscopic features of IgG4-associated inflammatory pseudotumor of ureter. (A) Fibroinflammatory lesion involves the entire ureteral wall and extends into periureteral adipose tissue (H&E stain, original magnification ×12.5). (B) Abundant plasma cells are intermixed with scattered histiocytes, fibroblasts and collagen fibers (H&E stain, original magnification ×400). (C) Obliterative phlebitis is identified in the periureteral adipose tissue (H&E stain, original magnification ×100). (D) A significant subset of plasma cells is positive for IgG4 (immunohistochemical stain, original magnification ×200). IgG4, immunoglobulin G4.
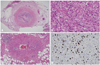
Fig. 5
Microscopic features of IgG4-related lymphadenopathy, type I. (A) Multicentric Castleman disease-like pattern with interfolicular expansion (H&E stain, ×40). (B) Perivascular sclerosis and hyalinized venules in lymphoid follicles are identified (H&E stain, ×100). (C) The interfollicular area is infiltrated by plasma cells intermixed with collagen fibers (H&E stain, ×400). (D) Many plasma cells are positive for IgG4 (immunohistochemical stain, ×200). IgG4, immunoglobulin G4.
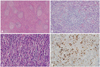
Table 1
Asian Diagnostic Criteria (Japan-Korea Consensus) for Autoimmune Pancreatitis (IgG4-related Sclerosing Pancreatitis)

References
1. Nirula A, Glaser SM, Kalled SL, Taylor FR. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr Opin Rheumatol. 2011. 23:119–124.

2. Meulenbroek AJ, Zeijlemaker WP. Human IgG Subclasses: useful diagnostic markers for immunocompetence [online]. 1996. Sanquin: Laboratory for Experimental and Clinical Immunology University of Amsterdam, the Netherlands;(http://www.xs4all.nl/_ednieuw/IgGsubclasses/subkl.htm).
3. Aucouturier P, Danon F, Daveau M, Guillou B, Sabbah A, Besson J, et al. Measurement of serum IgG4 levels by a competitive immunoenzymatic assay with monoclonal antibodies. J Immunol Methods. 1984. 74:151–162.

4. French MA. Serum IgG subclasses in normal adults. Monogr Allergy. 1986. 19:100–107.
5. Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009. 39:469–477.

6. Tao MH, Smith RI, Morrison SL. Structural features of human immunoglobulin G that determine isotype-specific differences in complement activation. J Exp Med. 1993. 178:661–667.

7. Brekke OH, Michaelsen TE, Aase A, Sandin RH, Sandlie I. Human IgG isotype-specific amino acid residues affecting complement-mediated cell lysis and phagocytosis. Eur J Immunol. 1994. 24:2542–2547.

8. Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998. 160:3555–3561.
9. Horner AA, Widhopf GF, Burger JA, Takabayashi K, Cinman N, Ronaghy A, et al. Immunostimulatory DNA inhibits IL-4-dependent IgE synthesis by human B cells. J Allergy Clin Immunol. 2001. 108:417–423.

10. Jones CC, Hamilton RG, Jordon RE. Subclass distribution of human IgG autoantibodies in pemphigus. J Clin Immunol. 1988. 8:43–49.

11. Futei Y, Amagai M, Ishii K, Kuroda-Kinoshita K, Ohya K, Nishikawa T. Predominant IgG4 subclass in autoantibodies of pemphigus vulgaris and foliaceus. J Dermatol Sci. 2001. 26:55–61.

12. Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005. 5:271–283.

13. Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease? Am J Dig Dis. 1961. 6:688–698.

14. Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995. 40:1561–1568.
16. Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, et al. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004. 445:552–563.

17. Aparisi L, Farre A, Gomez-Cambronero L, Martinez J, De Las Heras G, Corts J, et al. Antibodies to carbonic anhydrase and IgG4 levels in idiopathic chronic pancreatitis: relevance for diagnosis of autoimmune pancreatitis. Gut. 2005. 54:703–709.

18. Okazaki K, Uchida K, Ohana M, Nakase H, Uose S, Inai M, et al. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000. 118:573–581.

19. Deshpande V, Mino-Kenudson M, Brugge W, Lauwers GY. Autoimmune pancreatitis: more than just a pancreatic disease? A contemporary review of its pathology. Arch Pathol Lab Med. 2005. 129:1148–1154.

20. Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol. 2006. 41:613–625.

21. Kawa S, Ota M, Yoshizawa K, Horiuchi A, Hamano H, Ochi Y, et al. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology. 2002. 122:1264–1269.

22. Sood S, Fossard DP, Shorrock K. Chronic sclerosing pancreatitis in Sjögren's syndrome: a case report. Pancreas. 1995. 10:419–421.

23. Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991. 22:387–395.

24. Ectors N, Maillet B, Aerts R, Geboes K, Donner A, Borchard F, et al. Non-alcoholic duct destructive chronic pancreatitis. Gut. 1997. 41:263–268.

25. Yadav D, Notahara K, Smyrk TC, Clain JE, Pearson RK, Farnell MB, et al. Idiopathic tumefactive chronic pancreatitis: clinical profile, histology, and natural history after resection. Clin Gastroenterol Hepatol. 2003. 1:129–135.

26. Wakabayashi T, Kawaura Y, Satomura Y, Watanabe H, Motoo Y, Sawabu N. Long-term prognosis of duct-narrowing chronic pancreatitis: strategy for steroid treatment. Pancreas. 2005. 30:31–39.
27. Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003. 27:1119–1127.

28. Sah RP, Chari ST, Pannala R, Sugumar A, Clain JE, Levy MJ, et al. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology. 2010. 139:140–148.

29. Kamisawa T, Funata N, Hayashi Y. Lymphoplasmacytic sclerosing pancreatitis is a pancreatic lesion of IgG4-related systemic disease. Am J Surg Pathol. 2004. 28:1114.

30. Zhang L, Notohara K, Levy MJ, Chari ST, Smyrk TC. IgG4-positive plasma cell infiltration in the diagnosis of autoimmune pancreatitis. Mod Pathol. 2007. 20:23–28.

31. Cheuk W, Chan JK. IgG4-related sclerosing disease: a critical appraisal of an evolving clinicopathologic entity. Adv Anat Pathol. 2010. 17:303–332.
32. Deshpande V, Chicano S, Finkelberg D, Selig MK, Mino-Kenudson M, Brugge WR, et al. Autoimmune pancreatitis: a systemic immune complex mediated disease. Am J Surg Pathol. 2006. 30:1537–1545.

33. Mino-Kenudson M, Smyrk TC, Deshpande V, Fujisawa M, Shimizu M, Uehara T, et al. Autoimmune pancreatitis: West vs. East. Mod Pathol. 2008. 21:Suppl 1. A312.
34. Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006. 41:626–631.

35. Kim KP, Kim MH, Kim JC, Lee SS, Seo DW, Lee SK. Diagnostic criteria for autoimmune chronic pancreatitis revisited. World J Gastroenterol. 2006. 12:2487–2496.

36. Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, et al. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008. 43:403–408.

37. Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006. 4:1010–1016.

38. Frulloni L, Scattolini C, Falconi M, Zamboni G, Capelli P, Manfredi R, et al. Autoimmune pancreatitis: differences between the focal and diffuse forms in 87 patients. Am J Gastroenterol. 2009. 104:2288–2294.

39. Sugumar A, Klöppel G, Chari ST. Autoimmune pancreatitis: pathologic subtypes and their implications for its diagnosis. Am J Gastroenterol. 2009. 104:2308–2310.

40. Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001. 344:732–738.

41. Kamisawa T, Funata N, Hayashi Y, Tsuruta K, Okamoto A, Amemiya K, et al. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003. 52:683–687.

42. Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003. 38:982–984.

43. Sepehr A, Mino-Kenudson M, Ogawa F, Brugge WR, Deshpande V, Lauwers GY. IgG4+ to IgG+ plasma cells ratio of ampulla can help differentiate autoimmune pancreatitis from other "mass forming" pancreatic lesions. Am J Surg Pathol. 2008. 32:1770–1779.

44. Kamisawa T, Yoshiike M, Egawa N, Nakajima H, Tsuruta K, Okamoto A, et al. Chronic pancreatitis in the elderly in Japan. Pancreatology. 2004. 4:223–227.

45. Kim KP, Kim MH, Song MH, Lee SS, Seo DW, Lee SK. Autoimmune chronic pancreatitis. Am J Gastroenterol. 2004. 99:1605–1616.

46. Nishimori I, Tamakoshi A, Otsuki M. Research Committee on Intractable Diseases of the Pancreas, Ministry of Health, Labour, and Welfare of Japan. Prevalence of autoimmune pancreatitis in Japan from a nationwide survey in 2002. J Gastroenterol. 2007. 42:6–8.

47. Hardacre JM, Iacobuzio-Donahue CA, Sohn TA, Abraham SC, Yeo CJ, Lillemoe KD. Results of pancreaticoduodenectomy for lymphoplasmacytic sclerosing pancreatitis. Ann Surg. 2003. 237:853–858.

48. Abraham SC, Wilentz RE, Yeo CJ, Sohn TA, Cameron JL, Boitnott JK, et al. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: are they all 'chronic pancreatitis'? Am J Surg Pathol. 2003. 27:110–120.

49. Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 2010. 62:1755–1762.

50. Suda K, Nishimori I, Takase M, Oi I, Ogawa M. Autoimmune pancreatitis can be classified into early and advanced stages. Pancreas. 2006. 33:345–350.

51. Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, et al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004. 28:1193–1203.

52. Ito T, Nishimori I, Inoue N, Kawabe K, Gibo J, Arita Y, et al. Treatment for autoimmune pancreatitis: consensus on the treatment for patients with autoimmune pancreatitis in Japan. J Gastroenterol. 2007. 42:50–58.

53. Dhall D, Suriawinata AA, Tang LH, Shia J, Klimstra DS. Use of immunohistochemistry for IgG4 in the distinction of autoimmune pancreatitis from peritumoral pancreatitis. Hum Pathol. 2010. 41:643–652.

54. Kamisawa T, Okamoto A, Funata N. Clinicopathological features of autoimmune pancreatitis in relation to elevation of serum IgG4. Pancreas. 2005. 31:28–31.

55. Raina A, Krasinskas AM, Greer JB, Lamb J, Fink E, Moser AJ, et al. Serum immunoglobulin G fraction 4 levels in pancreatic cancer: elevations not associated with autoimmune pancreatitis. Arch Pathol Lab Med. 2008. 132:48–53.

56. Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007. 102:1646–1653.

57. Kitagawa S, Zen Y, Harada K, Sasaki M, Sato Y, Minato H, et al. Abundant IgG4-positive plasma cell infiltration characterizes chronic sclerosing sialadenitis (Küttner's tumor). Am J Surg Pathol. 2005. 29:783–791.

58. Wang WL, Farris AB, Lauwers GY, Deshpande V. Autoimmune pancreatitis-related cholecystitis: a morphologically and immunologically distinctive form of lymphoplasmacytic sclerosing cholecystitis. Histopathology. 2009. 54:829–836.

59. Chandan VS, Iacobuzio-Donahue C, Abraham SC. Patchy distribution of pathologic abnormalities in autoimmune pancreatitis: implications for preoperative diagnosis. Am J Surg Pathol. 2008. 32:1762–1769.

60. Moon SH, Kim MH, Park do H, Song TJ, Eum J, Lee SS, et al. IgG4 immunostaining of duodenal papillary biopsy specimens may be useful for supporting a diagnosis of autoimmune pancreatitis. Gastrointest Endosc. 2010. 71:960–966.

61. Geyer JT, Ferry JA, Harris NL, Stone JH, Zukerberg LR, Lauwers GY, et al. Chronic sclerosing sialadenitis (Küttner tumor) is an IgG4-associated disease. Am J Surg Pathol. 2010. 34:202–210.

62. Hamano H, Arakura N, Muraki T, Ozaki Y, Kiyosawa K, Kawa S. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol. 2006. 41:1197–1205.

63. Kitagawa S, Zen Y, Harada K, Sasaki M, Sato Y, Minato H, et al. Abundant IgG4-positive plasma cell infiltration characterizes chronic sclerosing sialadenitis (Küttner's tumor). Am J Surg Pathol. 2005. 29:783–791.

64. Zen Y, Nakanuma Y. IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol. 2010. 34:1812–1819.
65. Cheuk W, Yuen HK, Chan JK. Chronic sclerosing dacryoadenitis: part of the spectrum of IgG4-related Sclerosing disease? Am J Surg Pathol. 2007. 31:643–645.

66. Cheuk W, Yuen HK, Chu SY, Chiu EK, Lam LK, Chan JK. Lymphadenopathy of IgG4-related sclerosing disease. Am J Surg Pathol. 2008. 32:671–681.

67. Zen Y, Sawazaki A, Miyayama S, Notsumata K, Tanaka N, Nakanuma Y. A case of retroperitoneal and mediastinal fibrosis exhibiting elevated levels of IgG4 in the absence of sclerosing pancreatitis (autoimmune pancreatitis). Hum Pathol. 2006. 37:239–243.

68. Cheuk W, Lee KC, Chong LY, Yuen ST, Chan JK. IgG4-related Sclerosing disease: a potential new etiology of cutaneous pseudolymphoma. Am J Surg Pathol. 2009. 33:1713–1719.
69. Zen Y, Kitagawa S, Minato H, Kurumaya H, Katayanagi K, Masuda S, et al. IgG4-positive plasma cells in inflammatory pseudotumor (plasma cell granuloma) of the lung. Hum Pathol. 2005. 36:710–717.

70. Cheuk W, Chan AC, Lam WL, Chow SM, Crowley P, Lloydd R, et al. IgG4-related sclerosing mastitis: description of a new member of the IgG4-related sclerosing diseases. Am J Surg Pathol. 2009. 33:1058–1064.

71. Kasashima S, Zen Y, Kawashima A, Konishi K, Sasaki H, Endo M, et al. Inflammatory abdominal aortic aneurysm: close relationship to IgG4-related periaortitis. Am J Surg Pathol. 2008. 32:197–204.

72. Kakudo K, Li Y, Hirokawa M, Ozaki T. Diagnosis of Hashimoto's thyroiditis and IgG4-related sclerosing disease. Pathol Int. 2011. 61:175–183.

73. Okazaki K, Uchida K, Chiba T. Recent concept of autoimmune-related pancreatitis. J Gastroenterol. 2001. 36:293–302.

75. Saeki T, Saito A, Hiura T, Yamazaki H, Emura I, Ueno M, et al. Lymphoplasmacytic infiltration of multiple organs with immunoreactivity for IgG4: IgG4-related systemic disease. Intern Med. 2006. 45:163–167.

76. Shinji A, Sano K, Hamano H, Unno H, Fukushima M, Nakamura N, et al. Autoimmune pancreatitis is closely associated with gastric ulcer presenting with abundant IgG4-bearing plasma cell infiltration. Gastrointest Endosc. 2004. 59:506–511.

78. Bateman AC, Deheragoda MG. IgG4-related systemic sclerosing disease - an emerging and under-diagnosed condition. Histopathology. 2009. 55:373–383.

79. Sato Y, Notohara K, Kojima M, Takata K, Masaki Y, Yoshino T. IgG4-related disease: historical overview and pathology of hematological disorders. Pathol Int. 2010. 60:247–258.

80. Neild GH, Rodriguez-Justo M, Wall C, Connolly JO. Hyper-IgG4 disease: report and characterisation of a new disease. BMC Med. 2006. 4:23.

81. Deshpande V, Sainani NI, Chung RT, Pratt DS, Mentha G, Rubbia-Brandt L, et al. IgG4-associated cholangitis: a comparative histological and immunophenotypic study with primary sclerosing cholangitis on liver biopsy material. Mod Pathol. 2009. 22:1287–1295.

82. Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008. 134:706–715.

83. Kamisawa T, Nakajima H, Egawa N, Funata N, Tsuruta K, Okamoto A. IgG4-related sclerosing disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis and retroperitoneal fibrosis with lymphadenopathy. Pancreatology. 2006. 6:132–137.

84. Nakazawa T, Ohara H, Sano H, Ando T, Aoki S, Kobayashi S, et al. Clinical differences between primary sclerosing cholangitis and sclerosing cholangitis with autoimmune pancreatitis. Pancreas. 2005. 30:20–25.
85. Nakanuma Y, Zen Y. Pathology and immunopathology of immunoglobulin G4-related sclerosing cholangitis: the latest addition to the sclerosing cholangitis family. Hepatol Res. 2007. 37:S478–S486.

86. Nakanuma Y, Harada K, Katayanagi K, Tsuneyama K, Sasaki M. Definition and pathology of primary sclerosing cholangitis. J Hepatobiliary Pancreat Surg. 1999. 6:333–342.

87. Oseini AM, Chaiteerakij R, Shire AM, Ghazale A, Kaiya J, Moser CD, et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology. 2011. 06. 14. doi:10.1002/hep.24487 [Epub ahead of print].

88. Erkelens GW, Vleggaar FP, Lesterhuis W, van Buuren HR, van der Werf SD. Sclerosing pancreato-cholangitis responsive to steroid therapy. Lancet. 1999. 354:43–44.

89. Nakazawa T, Ohara H, Yamada T, Ando H, Sano H, Kajino S, et al. Atypical primary sclerosing cholangitis cases associated with unusual pancreatitis. Hepatogastroenterology. 2001. 48:625–630.
90. Kojima E, Kimura K, Noda Y, Kobayashi G, Itoh K, Fujita N. Autoimmune pancreatitis and multiple bile duct strictures treated effectively with steroid. J Gastroenterol. 2003. 38:603–607.

91. Kuroiwa T, Suda T, Takahashi T, Hirono H, Natsui M, Motoyama H, et al. Bile duct involvement in a case of autoimmune pancreatitis successfully treated with an oral steroid. Dig Dis Sci. 2002. 47:1810–1816.
92. Mendes FD, Jorgensen R, Keach J, Katzmann JA, Smyrk T, Donlinger J, et al. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006. 101:2070–2075.

93. Webster GJ, Pereira SP, Chapman RW. Autoimmune pancreatitis/IgG4-associated cholangitis and primary sclerosing cholangitis--overlapping or separate diseases? J Hepatol. 2009. 51:398–402.

94. Abraham SC, Cruz-Correa M, Argani P, Furth EE, Hruban RH, Boitnott JK. Lymphoplasmacytic chronic cholecystitis and biliary tract disease in patients with lymphoplasmacytic sclerosing pancreatitis. Am J Surg Pathol. 2003. 27:441–451.

95. Jessurun J, Bolio-Solis A, Manivel JC. Diffuse lymphoplasmacytic acalculous cholecystitis: a distinctive form of chronic cholecystitis associated with primary sclerosing cholangitis. Hum Pathol. 1998. 29:512–517.

96. Zen Y, Fujii T, Sato Y, Masuda S, Nakanuma Y. Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Mod Pathol. 2007. 20:884–894.

97. Kanno A, Satoh K, Kimura K, Masamune A, Asakura T, Unno M, et al. Autoimmune pancreatitis with hepatic inflammatory pseudotumor. Pancreas. 2005. 31:420–423.

98. Travis WD, Elisabeth B, Muller-Hermelink HK, Harris CC, editors. Pathology and Genetics of Tumours of the Lung, Pleural, Thymus and Heart. 2004. Lyon: IARC Press.
99. Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. 2001. 25:721–731.
100. Umemura T, Zen Y, Hamano H, Kawa S, Nakanuma Y, Kiyosawa K. Immunoglobin G4-hepatopathy: association of immunoglobin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology. 2007. 46:463–471.

101. Umemura T, Zen Y, Hamano H, Ichijo T, Kawa S, Nakanuma Y, et al. IgG4 associated autoimmune hepatitis: a differential diagnosis for classical autoimmune hepatitis. Gut. 2007. 56:1471–1472.

102. Kuttner H. Uber entzundiche Tumoren der Submaaxillar-speicheldruse. Bruns Beitr Klin Chir. 1896. 15:815–834.
103. Pilch BZ. Head and Neck Surgical Pathology. 2001. Philadelphia: Lippincott Williams and Wilkin.
104. Tiemann M, Teymoortash A, Schrader C, Werner JA, Parwaresch R, Seifert G, et al. Chronic sclerosing sialadenitis of the submandibular gland is mainly due to a T lymphocyte immune reaction. Mod Pathol. 2002. 15:845–852.

105. Cheuk W, Chan JK. Kuttner tumor of the submandibular gland: fine-needle aspiration cytologic findings of seven cases. Am J Clin Pathol. 2002. 117:103–108.
106. Kamisawa T, Nakajima H, Hishima T. Close correlation between chronic sclerosing sialadenitis and immunoglobulin G4. Intern Med J. 2006. 36:527–529.

107. Chan JK. Kuttner tumor (chronic sclerosing sialadenitis) of the submandibular gland: an underrecognized entity. Adv Anat Pathol. 1998. 5:239–251.

108. Yamamoto M, Takahashi H, Sugai S, Imai K. Clinical and pathological characteristics of Mikulicz's disease (IgG4-related plasmacytic exocrinopathy). Autoimmun Rev. 2005. 4:195–200.

109. Masaki Y, Dong L, Kurose N, Kitagawa K, Morikawa Y, Yamamoto M, et al. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis. 2009. 68:1310–1315.

110. Yamamoto M, Takahashi H, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, et al. A new conceptualization for Mikulicz's disease as an IgG4-related plasmacytic disease. Mod Rheumatol. 2006. 16:335–340.

111. Yamamoto H, Yamaguchi H, Aishima S, Oda Y, Kohashi K, Oshiro Y, et al. Inflammatory myofibroblastic tumor versus IgG4-related sclerosing disease and inflammatory pseudotumor: a comparative clinicopathologic study. Am J Surg Pathol. 2009. 33:1330–1340.

112. Comings DE, Skubi KB, VanEyes J, Motulsky AG. Familial multifocal fibrosclerosis. Findings suggesting that retroperitoneal fibrosis, mediastinal fibrosis, sclerosing cholangitis, Riedel's thyroiditis, and pseudotumor of the orbit may be different manifestations of a single disease. Ann Intern Med. 1967. 66:884–892.
113. Klisnick A, Fourcade J, Ruivard M, Baud O, Souweine B, Boyer L, et al. Combined idiopathic retroperitoneal and mediastinal fibrosis with pericardial involvement. Clin Nephrol. 1999. 52:51–55.
114. Graal MB, Lustermans FA. A patient with combined mediastinal, mesenteric and retroperitoneal fibrosis. Neth J Med. 1994. 44:214–219.
115. Owen K, Lane H, Jones MK. Multifocal fibrosclerosis: a case of thyroiditis and bilateral lacrimal gland involvement. Thyroid. 2001. 11:1187–1190.

116. Morris WR, Haik BG, Osborn D, Fleming JC. Intraocular involvement in multifocal fibrosclerosis. Ophthalmology. 2000. 107:962–966.

117. Levey JM, Mathai J. Diffuse pancreatic fibrosis: an uncommon feature of multifocal idiopathic fibrosclerosis. Am J Gastroenterol. 1998. 93:640–642.

118. Fukuda W, Kimura M, Akaogi T, Sako M, Ohiwa K, Yamamoto Y, et al. Multifocal fibrosclerosis: retroperitoneal fibrosis associated with a suprasellar tumor and pachymeningitis. Intern Med. 2003. 42:1006–1010.

119. Pacini D, Leone O, Turci S, Camurri N, Giunchi F, Martinelli GN, et al. Incidence, etiology, histologic findings, and course of thoracic inflammatory aortopathies. Ann Thorac Surg. 2008. 86:1518–1523.

120. Miller DV, Isotalo PA, Weyand CM, Edwards WD, Aubry MC, Tazelaar HD. Surgical pathology of noninfectious ascending aortitis: a study of 45 cases with emphasis on an isolated variant. Am J Surg Pathol. 2006. 30:1150–1158.

121. Burke AP, Tavora F, Narula N, Tomaszewski JE, Virmani R. Aortitis and ascending aortic aneurysm: description of 52 cases and proposal of a histologic classification. Hum Pathol. 2008. 39:514–526.

122. Dehner LP, Coffin CM. Idiopathic fibrosclerotic disorders and other inflammatory pseudotumors. Semin Diagn Pathol. 1998. 15:161–173.
123. Clark A, Zeman RK, Choyke PL, White EM, Burrell MI, Grant EG, et al. Pancreatic pseudotumors associated with multifocal idiopathic fibrosclerosis. Gastrointest Radiol. 1988. 13:30–32.

124. Hamano H, Kawa S, Ochi Y, Unno H, Shiba N, Wajiki M, et al. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002. 359:1403–1404.

125. Zen Y, Onodera M, Inoue D, Kitao A, Matsui O, Nohara T, et al. Retroperitoneal fibrosis: a clinicopathologic study with respect to immunoglobulin G4. Am J Surg Pathol. 2009. 33:1833–1839.

126. Chen TS, Montgomery EA. Are tumefactive lesions classified as sclerosing mesenteritis a subset of IgG4-related sclerosing disorders? J Clin Pathol. 2008. 61:1093–1097.

127. Matsumoto Y, Kasashima S, Kawashima A, Sasaki H, Endo M, Kawakami K, et al. A case of multiple immunoglobulin G4-related periarteritis: a tumorous lesion of the coronary artery and abdominal aortic aneurysm. Hum Pathol. 2008. 39:975–980.

128. Sakata N, Tashiro T, Uesugi N, Kawara T, Furuya K, Hirata Y, et al. IgG4-positive plasma cells in inflammatory abdominal aortic aneurysm: the possibility of an aortic manifestation of IgG4-related sclerosing disease. Am J Surg Pathol. 2008. 32:553–559.

129. Qian Q, Kashani KB, Miller DV. Ruptured abdominal aortic aneurysm related to IgG4 periaortitis. N Engl J Med. 2009. 361:1121–1123.

130. Ito H, Kaizaki Y, Noda Y, Fujii S, Yamamoto S. IgG4-related inflammatory abdominal aortic aneurysm associated with autoimmune pancreatitis. Pathol Int. 2008. 58:421–426.

131. Ishida M, Hotta M, Kushima R, Asai T, Okabe H. IgG4-related inflammatory aneurysm of the aortic arch. Pathol Int. 2009. 59:269–273.

132. Stone JH, Khosroshahi A, Deshpande V, Stone JR. IgG4-related systemic disease accounts for a significant proportion of thoracic lymphoplasmacytic aortitis cases. Arthritis Care Res (Hoboken). 2010. 62:316–322.

133. Komatsu K, Hamano H, Ochi Y, Takayama M, Muraki T, Yoshizawa K, et al. High prevalence of hypothyroidism in patients with autoimmune pancreatitis. Dig Dis Sci. 2005. 50:1052–1057.

134. Li Y, Bai Y, Liu Z, Ozaki T, Taniguchi E, Mori I, et al. Immunohistochemistry of IgG4 can help subclassify Hashimoto's autoimmune thyroiditis. Pathol Int. 2009. 59:636–641.

135. Li Y, Nishihara E, Hirokawa M, Taniguchi E, Miyauchi A, Kakudo K. Distinct clinical, serological, and sonographic characteristics of hashimoto's thyroiditis based with and without IgG4-positive plasma cells. J Clin Endocrinol Metab. 2010. 95:1309–1317.

136. Drieskens O, Blockmans D, Van den Bruel A, Mortelmans L. Riedel's thyroiditis and retroperitoneal fibrosis in multifocal fibrosclerosis: positron emission tomographic findings. Clin Nucl Med. 2002. 27:413–415.

137. Dahlgren M, Khosroshahi A, Nielsen GP, Deshpande V, Stone JH. Riedel's thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis Care Res (Hoboken). 2010. 62:1312–1318.

138. Zhang L, Smyrk TC. Autoimmune pancreatitis and IgG4-related systemic diseases. Int J Clin Exp Pathol. 2010. 3:491–504.
139. Shrestha B, Sekiguchi H, Colby TV, Graziano P, Aubry MC, Smyrk TC, et al. Distinctive pulmonary histopathology with increased IgG4-positive plasma cells in patients with autoimmune pancreatitis: report of 6 and 12 cases with similar histopathology. Am J Surg Pathol. 2009. 33:1450–1462.

140. Zen Y, Inoue D, Kitao A, Onodera M, Abo H, Miyayama S, et al. IgG4-related lung and pleural disease: a clinicopathologic study of 21 cases. Am J Surg Pathol. 2009. 33:1886–1893.

141. Yamashita K, Haga H, Kobashi Y, Miyagawa-Hayashino A, Yoshizawa A, Manabe T. Lung involvement in IgG4-related lymphoplasmacytic vasculitis and interstitial fibrosis: report of 3 cases and review of the literature. Am J Surg Pathol. 2008. 32:1620–1626.

142. Kobayashi H, Shimokawaji T, Kanoh S, Motoyoshi K, Aida S. IgG4-positive pulmonary disease. J Thorac Imaging. 2007. 22:360–362.

143. Zen Y, Kasahara Y, Horita K, Miyayama S, Miura S, Kitagawa S, et al. Inflammatory pseudotumor of the breast in a patient with a high serum IgG4 level: histologic similarity to sclerosing pancreatitis. Am J Surg Pathol. 2005. 29:275–278.

144. Watson SJ, Jenkins DA, Bellamy CO. Nephropathy in IgG4-related systemic disease. Am J Surg Pathol. 2006. 30:1472–1477.

145. Cornell LD, Chicano SL, Deshpande V, Collins AB, Selig MK, Lauwers GY, et al. Pseudotumors due to IgG4 immune-complex tubulointerstitial nephritis associated with autoimmune pancreatocentric disease. Am J Surg Pathol. 2007. 31:1586–1597.

146. Dhobale S, Bedetti C, Killian P, Ilyas M, Liput J, Jasnosz K, et al. IgG4 related sclerosing disease with multiple organ involvements and response to corticosteroid treatment. J Clin Rheumatol. 2009. 15:354–357.

147. Takeda S, Haratake J, Kasai T, Takaeda C, Takazakura E. IgG4-associated idiopathic tubulointerstitial nephritis complicating autoimmune pancreatitis. Nephrol Dial Transplant. 2004. 19:474–476.

148. Saeki T, Nishi S, Ito T, Yamazaki H, Miyamura S, Emura I, et al. Renal lesions in IgG4-related systemic disease. Intern Med. 2007. 46:1365–1371.

149. Kuroda N, Nakamura S, Miyazaki K, Inoue K, Ohara M, Mizuno K, et al. Chronic sclerosing pyelitis with an increased number of IgG4-positive plasma cells. Med Mol Morphol. 2009. 42:236–238.

150. Kim SA, Lee SR, Huh J, Shen SS, Ro JY. IgG4-associated inflammatory pseudotumor of ureter: clinicopathologic and immunohistochemical study of 3 cases. Hum Pathol. 2011. 42:1178–1184.

151. Hori M, Makita N, Andoh T, Takiyama H, Yajima Y, Sakatani T, et al. Long-term clinical course of IgG4-related systemic disease accompanied by hypophysitis. Endocr J. 2010. 57:485–492.

152. Shimatsu A, Oki Y, Fujisawa I, Sano T. Pituitary and stalk lesions (infundibulo-hypophysitis) associated with immunoglobulin G4-related systemic disease: an emerging clinical entity. Endocr J. 2009. 56:1033–1041.

153. Wong S, Lam WY, Wong WK, Lee KC. Hypophysitis presented as inflammatory pseudotumor in immunoglobulin G4-related systemic disease. Hum Pathol. 2007. 38:1720–1723.

154. Osawa S, Ogawa Y, Watanabe M, Tominaga T. Hypophysitis presenting with atypical rapid deterioration: with special reference to immunoglobulin G4-related disease-case report-. Neurol Med Chir (Tokyo). 2009. 49:622–625.

155. Tanabe T, Tsushima K, Yasuo M, Urushihata K, Hanaoka M, Koizumi T, et al. IgG4-associated multifocal systemic fibrosis complicating sclerosing sialadenitis, hypophysitis, and retroperitoneal fibrosis, but lacking pancreatic involvement. Intern Med. 2006. 45:1243–1247.

156. Yamamoto M, Takahashi H, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, et al. A case of Mikulicz's disease (IgG4-related plasmacytic disease) complicated by autoimmune hypophysitis. Scand J Rheumatol. 2006. 35:410–411.

157. Chan SK, Cheuk W, Chan KT, Chan JK. IgG4-related sclerosing pachymeningitis: a previously unrecognized form of central nervous system involvement in IgG4-related sclerosing disease. Am J Surg Pathol. 2009. 33:1249–1252.
158. Kupersmith MJ, Martin V, Heller G, Shah A, Mitnick HJ. Idiopathic hypertrophic pachymeningitis. Neurology. 2004. 62:686–694.

160. Lui PC, Fan YS, Wong SS, Chan AN, Wong G, Chau TK, et al. Inflammatory pseudotumors of the central nervous system. Hum Pathol. 2009. 40:1611–1617.

161. Sato Y, Kojima M, Takata K, Morito T, Asaoku H, Takeuchi T, et al. Systemic IgG4-related lymphadenopathy: a clinical and pathologic comparison to multicentric Castleman's disease. Mod Pathol. 2009. 22:589–599.

162. Sugimoto T, Morita Y, Isshiki K, Yamamoto T, Uzu T, Kashiwagi A, et al. Constrictive pericarditis as an emerging manifestation of hyper-IgG4 disease. Int J Cardiol. 2008. 130:e100–e101.

163. Uehara T, Hamano H, Kawakami M, Koyama M, Kawa S, Sano K, et al. Autoimmune pancreatitis-associated prostatitis: distinct clinicopathological entity. Pathol Int. 2008. 58:118–125.

164. Taniguchi T, Kobayashi H, Fukui S, Ogura K, Saiga T, Okamoto M. A case of multifocal fibrosclerosis involving posterior mediastinal fibrosis, retroperitoneal fibrosis, and a left seminal vesicle with elevated serum IgG4. Hum Pathol. 2006. 37:1237–1239. author reply 1239.

165. Ikeda R, Awataguchi T, Shoji F, Oshima T. A case of paranasal sinus lesions in IgG4-related sclerosing disease. Otolaryngol Head Neck Surg. 2010. 142:458–459.

166. Ishida M, Hotta M, Kushima R, Shibayama M, Shimizu T, Okabe H. Multiple IgG4-related sclerosing lesions in the maxillary sinus, parotid gland and nasal septum. Pathol Int. 2009. 59:670–675.

167. Cheuk W, Tam FK, Chan AN, Luk IS, Yuen AP, Chan WK, et al. Idiopathic cervical fibrosis--a new member of IgG4-related sclerosing diseases: report of 4 cases, 1 complicated by composite lymphoma. Am J Surg Pathol. 2010. 34:1678–1685.

168. Lopes J, Hochwald SN, Lancia N, Dixon LR, Ben-David K. Autoimmune esophagitis: IgG4-related tumors of the esophagus. J Gastrointest Surg. 2010. 14:1031–1034.

169. Martel M, Cheuk W, Lombardi L, Lifschitz-Mercer B, Chan JK, Rosai J. Sclerosing angiomatoid nodular transformation (SANT): report of 25 cases of a distinctive benign splenic lesion. Am J Surg Pathol. 2004. 28:1268–1279.
170. Nagai Y, Hayama N, Kishimoto T, Furuya M, Takahashi Y, Otsuka M, et al. Predominance of IgG4+ plasma cells and CD68 positivity in sclerosing angiomatoid nodular transformation (SANT). Histopathology. 2008. 53:495–498.

171. Kashiwagi S, Kumasaka T, Bunsei N, Fukumura Y, Yamasaki S, Abe K, et al. Detection of Epstein-Barr virus-encoded small RNA-expressed myofibroblasts and IgG4-producing plasma cells in sclerosing angiomatoid nodular transformation of the spleen. Virchows Arch. 2008. 453:275–282.

172. Kojima M, Miyawaki S, Takada S, Kashiwabara K, Igarashi T, Nakamura S. Lymphoplasmacytic infiltrate of regional lymph nodes in Kuttner's tumor (chronic sclerosing sialadenitis): a report of 3 cases. Int J Surg Pathol. 2008. 16:263–268.

173. Boulanger E, Fuentes V, Meignin V, Mougenot B, Labaume S, Gouilleux-Gruart V, et al. Polyclonal IgG4 hypergammaglobulinemia associated with plasmacytic lymphadenopathy, anemia and nephropathy. Ann Hematol. 2006. 85:833–840.

174. Kojima M, Nakamura N, Tsukamoto N, Otuski Y, Shimizu K, Itoh H, et al. Clinical implications of idiopathic multicentric castleman disease among Japanese: a report of 28 cases. Int J Surg Pathol. 2008. 16:391–398.

175. Nishimoto N, Kanakura Y, Aozasa K, Johkoh T, Nakamura M, Nakano S, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005. 106:2627–2632.

176. Kojima M, Hosomura Y, Itoh H, Johshita T, Yoshida K, Nakamura S, et al. Reactive proliferative lesions in lymph nodes from rheumatoid arthritis patients. A clinicopathological and immunohistological study. Acta Pathol Jpn. 1990. 40:249–254.

177. Kojima M, Nakamura S, Morishita Y, Itoh H, Yoshida K, Ohno Y, et al. Reactive follicular hyperplasia in the lymph node lesions from systemic lupus erythematosus patients: a clinicopathological and immunohistological study of 21 cases. Pathol Int. 2000. 50:304–312.

178. Koo CH, Nathwani BN, Winberg CD, Hill LR, Rappaport H. Atypical lymphoplasmacytic and immunoblastic proliferation in lymph nodes of patients with autoimmune disease (autoimmune-disease-associated lymphadenopathy). Medicine (Baltimore). 1984. 63:274–290.

179. Ioachim HL, Medeiros LJ. Ioachim's Lymph Node Pathology. 2009. 4th ed. Philadelphia, PA: Lippincott, Williams & Wilkins;186–189.
180. Moran CA, Suster S, Abbondanzo SL. Inflammatory pseudotumor of lymph nodes: a study of 25 cases with emphasis on morphological heterogeneity. Hum Pathol. 1997. 28:332–338.

181. Cheuk W, Yuen HK, Chan AC, Shih LY, Kuo TT, Ma MW, et al. Ocular adnexal lymphoma associated with IgG4+ chronic sclerosing dacryoadenitis: a previously undescribed complication of IgG4-related sclerosing disease. Am J Surg Pathol. 2008. 32:1159–1167.

182. Takahashi N, Ghazale AH, Smyrk TC, Mandrekar JN, Chari ST. Possible association between IgG4-associated systemic disease with or without autoimmune pancreatitis and non-Hodgkin lymphoma. Pancreas. 2009. 38:523–526.

183. Motosugi U, Ichikawa T, Yamaguchi H, Nakazawa T, Katoh R, Itakura J, et al. Small invasive ductal adenocarcinoma of the pancreas associated with lymphoplasmacytic sclerosing pancreatitis. Pathol Int. 2009. 59:744–747.

184. Witkiewicz AK, Kennedy EP, Kennyon L, Yeo CJ, Hruban RH. Synchronous autoimmune pancreatitis and infiltrating pancreatic ductal adenocarcinoma: case report and review of the literature. Hum Pathol. 2008. 39:1548–1551.

185. Kamisawa T, Chen PY, Tu Y, Nakajima H, Egawa N, Tsuruta K, et al. Pancreatic cancer with a high serum IgG4 concentration. World J Gastroenterol. 2006. 12:6225–6228.

186. Inoue H, Miyatani H, Sawada Y, Yoshida Y. A case of pancreas cancer with autoimmune pancreatitis. Pancreas. 2006. 33:208–209.

187. Joo M, Chang SH, Kim H, Gardner JM, Ro JY. Primary gastrointestinal clear cell sarcoma: report of 2 cases, one case associated with IgG4-related sclerosing disease, and review of literature. Ann Diagn Pathol. 2009. 13:30–35.

188. Gill J, Angelo N, Yeong ML, McIvor N. Salivary duct carcinoma arising in IgG4-related autoimmune disease of the parotid gland. Hum Pathol. 2009. 40:881–886.

189. Zen Y, Fujii T, Harada K, Kawano M, Yamada K, Takahira M, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007. 45:1538–1546.

190. Chang MC, Chang YT, Wei SC, Kuo CH, Liang PC, Wong JM. Autoimmune pancreatitis associated with high prevalence of gastric ulcer independent of Helicobacter pylori infection status. Pancreas. 2009. 38:442–446.

191. Kountouras J, Zavos C, Chatzopoulos D. A concept on the role of Helicobacter pylori infection in autoimmune pancreatitis. J Cell Mol Med. 2005. 9:196–207.

192. Guarneri F, Guarneri C, Benvenga S. Helicobacter pylori and autoimmune pancreatitis: role of carbonic anhydrase via molecular mimicry? J Cell Mol Med. 2005. 9:741–744.

193. Okazaki K, Uchida K, Fukui T. Recent advances in autoimmune pancreatitis: concept, diagnosis, and pathogenesis. J Gastroenterol. 2008. 43:409–418.

194. Tanaka A, Moriyama M, Nakashima H, Miyake K, Hayashida JN, Maehara T, et al. Th2 andregulatory immune reactions contributes to IgG4 production and the initiation of Mikulicz's disease. Arthritis Rheum. 2011. 09. 06. doi: 10.1002/art.33320 [Epub ahead of print].
195. Koyabu M, Uchida K, Miyoshi H, Sakaguchi Y, Fukui T, Ikeda H, et al. Analysis of regulatory T cells and IgG4-positive plasma cells among patients of IgG4-related sclerosing cholangitis and autoimmune liver diseases. J Gastroenterol. 2010. 45:732–741.

196. Nakashima H, Miyake K, Moriyama M, Tanaka A, Watanabe M, Abe Y, et al. An amplification of IL-10 and TGF-beta in patients with IgG4-related tubulointerstitial nephritis. Clin Nephrol. 2010. 73:385–391.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download