Abstract
Radiation-induced arterial disease is caused by significant atherosclerosis in the circumjacent vessels being irradiated. Even though this has been recognized as survival of cancer patients treated with radiotherapy improves, it is a problem that is often under-reported. We present a case of chronic thromboembolic occlusion of right common iliac artery in a 53-year-old woman who was treated with radiation therapy for cervical cancer 13 years ago. We initially performed percutaneous transluminal angioplasty with thrombolytic therapy, but had to cease thrombolytic therapy due to upper gastrointestinal bleeding of Dieulafoy's lesion, nevertheless, achieved good results after revascularization by Fogarty embolectomy.
Radiation-induced arterial disease causes long-term complications such as stenosis, thrombosis, pseudoaneurysm and rupture.1-4 These complications arise several years after radiation therapy.5,6 Stenoses and occlusions are more often reported than ruptures and pseudoaneurysm, but remain very rare.7,8 Treatment of such lesion is difficult for several reasons. Here, we report our experience in the management of complication after radiation therapy for cervical cancer.
In June 2009, a non-smoking 53-year-old woman, without lipid metabolic disorders or hypertension, was admitted with complaints of progressive claudication in the right thigh and calf at 100 yards over a period of 2 years, and numbness and tingling in the right ankle over 1 month. She was treated for a squamous carcinoma of the cervix with metastasis of right external iliac lymph nodes, stage IIB, and underwent transvaginal hysterectomy and postoperative radiotherapy 13 years ago. Radiotherapy consisted of 5400 cGy external radiotherapy, intrapelvic optimal dose of 5040 cGy and booster dose of 360 cGy for metastatic lymph nodes with 10 MV energy photobeams using semiextended field, and she was treated daily with 5 sessions per week over 7 weeks and 180 cGy per fraction. The patient underwent small bowel resection due to bowel ischemia 2 years ago.
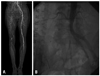
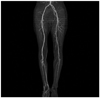
Clinical examination revealed absence of right femoral and distal pulses. The initial laboratory data showed hemoglobin of 10.1 g/dL, leukocyte 9060/µL with 80.6% neutrophils, blood urea nitrogen 19.8 mg/dL, creatinine 0.78 mg/dL, total cholesterol 118 mg/dL, triglyseride 62 mg/dL, high density lipoprotein 40 mg/dL, and low density lipoprotein 67 mg/dL. Laboratory data associated coagulation was within normal range (PT 12.6 sec, aPTT 25.5 sec, protein C 124%, protein S 89%, C3 90.0 mg/dL, C4 27.9 mg/dL). Autoantibody tests for rheumatoid factor, lupus anticoagulant, anticardiolipin, anti ds-DNA, and P-ANCA/C-ANCA were negative. The ankle-brachial index (ABI) on the right was 0.12, while that on the left was 1.13. A lower extremity computed tomographic angiography (CTA) showed chronic thromboemblic occlusion extending from the right common iliac artery to the popliteal artery (Fig. 1A). Diagnostic transbrachial arteriography showed same result as that with CT angiography (Fig. 1B).
Initially, the patient was treated by a percutaneous transluminal angioplasty (PTA) with thrombolytic therapy. According to our standard protocol, urokinase (UK) 300000 units were injected via infusion catheter and 200000 units were injected via sheath, and infusion of 80000 units per hour and heparin via sheath was kept continuously. Twenty hours after initiating thrombolytic therapy, we observed upper gastrointestinal bleeding due to Dieulafoy's lesion at the duodenal bulb. We, therefore, performed therapeutic endoscopy, epinephrine injection and hemoclipping, and discontinued injection of UK and heparin. Follow up angiography revealed improved flow of common iliac artery (CIA), but thromboembolic occlusion remained. We performed Redo-Fogarty embolectomy and found 10×0.5 cm sized organizing thrombi via the right common femoral artery incision site (Fig. 2). After the embolectomy, CTA revealed improved flow of the lesion, and the right ABI rose to 0.89 (Fig. 3). The patient was discharged with medication of aspirin 100 mg once daily (o.d.), clopidogrel 75 mg o.d., beraprost 40 µg o.d. and warfarin 2.5 mg o.d. Since then, there was one episode of treatment with PTA at the proximal portion of right superficial artery. With medical treatment, her lower limbs remain asymptomatic.
Radiation-induced arterial disease which complicates radiation therapy is rare. Radiation damage to arteries occurs initially with an injury to the extremely radiosensitive endothelial cell layer and then causes prolonged effects of peripheral fibrosis and accelerated atherosclerosis, which occur over the course of decades.9,10 The manifestation of atherosclerotic process in these vessels includes stenosis, thrombosis, and aneurismal dilatation.1-4 Radiation-induced arterial disease of aortoiliac arteries has been known to most commonly occur as a consequence of treatment for rectal, urologic, or gynecologic cancer. Establishing a presumptive diagnosis of radiation-induced occlusive arterial disease is based on the clinical history and angiographic appearance of lesion. Evidence of occlusive disease within the irradiated field with relative sparing of non-irradiated arteries is highly suggestive of radiation-induced occlusive arterial disease.11 However, other extremely rare cases of arterial occlusion are not limited to radiation field, because coagulation abnormalities have previously been presented in patients who had been treated for gynecologic cancer.12 In the present case, the patient complained of intermittent claudication in the right thigh and calf, and had received postoperative radiotherapy in the pelvic area for cervical cancer 13 years ago. She had no other cardiovascular risk factor and arterial occlusive diseases, and never smoked. Laboratory data associated coagulation was within normal range, and autoantibody tests were all negative. Considering all the factors, therefore, we concluded the diagnosis of radiation-induced arterial occlusive disease. The history of small bowel resection due to bowel ischemia might have been associated with previous radiation therapy.
The treatment of arterial lesion related to prior radiation is recommended for indications similar to atherosclerotic occlusive lesion, unless there is a poor long-term prognosis associated with malignancy.10 A variety of endovascular and surgical procedures may be used to treat radiation-induced occlusive disease in the aortoiliac artery.10 The presence of sclerosis in surrounding tissues and increased risk of postoperative infection mandate the use of autogenous conduits as well when possible.13,14 Recently, advanced percutaneous intervention has emerged as the treatment of choice for these lesions, however, the length of diseased artery and the circumjacent fibrosis limit its efficacy.11 In our case, we initially considered thrombolytic therapy because of chronic thromboemblic total occlusion affecting from the right CIA to the popliteal artery with diminutive caliber of remaining arteries. However, we had to stop thrombolytic therapy due to upper gastrointestinal bleeding, and could not inject enough dosage of UK via infusion catheter and sheath. Nevertheless, we actually achieved good results after Fogarty embolectomy.
Radiation-induced arterial disease is coordinative to accelerated atherosclerosis, and should be considered as a major risk factor for the development of ischemic syndromes in the irradiated field.15 Development of this disorder is related to the delivery technique of radiation, the dose of radiation, and the extent of exposed vasculature. Preventive measures should, therefore, be aimed at accurate limitation of the irradiated field, avoidance of overdoses, and reversal of associated atherosclerotic risk factors. Early observation and detection should also be needed for cancer survivors who previously received radiation therapy.
Figures and Tables
References
1. McCready RA, Hyde GL, Bivins BA, Mattingly SS, Griffen WO Jr. Radiation-induced arterial injuries. Surgery. 1983. 93:306–312.
2. Hughes WF, Carson CL, Laffaye HA. Subclavian artery occlusion 42 years after mastectomy and radiotherapy. Am J Surg. 1984. 147:698–700.

3. Har-Shai Y, Schein M, Molek AD, Peled IJ, Best LA. Ruptured mycotic aneurysm of the subclavian artery after irradiation. A case report. Eur J Surg. 1993. 159:59–60.
4. Ross HB, Sales JE. Post-irradiation femoral aneurysm treated by iliopopliteal by-pass via the obturator foramen. Br J Surg. 1972. 59:400–405.

5. de Baere T, Ousehal A, Kuoch V, Sapoval M, Lagrange C, Roche A. Endovascular management of bleeding iliac artery pseudoaneurysms complicating radiation therapy for pelvic malignancies. AJR Am J Roentgenol. 1998. 170:349–353.

6. Pettersson F, Swedenborg J. Atherosclerotic occlusive disease after radiation for pelvic malignancies. Acta Chir Scand. 1990. 156:367–371.
7. Mellière D, Becquemin JP, Berrahal D, Desgranges P, Cavillon A. Management of radiation-induced occlusive arterial disease: a reassessment. J Cardiovasc Surg (Torino). 1997. 38:261–269.
8. Katras T, Baltazar U, Colvett K, Rush D, Dunn J, Stanton P Jr. Radiation-related arterial disease. Am Surg. 1999. 65:1176–1179.
9. Butler MJ, Lane RH, Webster JH. Irradiation injury to large arteries. Br J Surg. 1980. 67:341–343.

10. Modrall JG, Sadjadi J. Early and late presentations of radiation arteritis. Semin Vasc Surg. 2003. 16:209–214.

11. Jurado JA, Bashir R, Burket MW. Radiation-induced peripheral artery disease. Catheter Cardiovasc Interv. 2008. 72:563–568.

12. Levenback C, Burke TW, Rubin SC, Curtin JP, Wharton JT. Arterial occlusion complicating treatment of gynecologic cancer: a case series. Gynecol Oncol. 1996. 63:40–46.

13. Andros G, Schneider PA, Harris RW, Dulawa LB, Oblath RW, Salles-Cunha SX. Management of arterial occlusive disease following radiation therapy. Cardiovasc Surg. 1996. 4:135–142.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download