Abstract
Purpose
The cytochrome P450 2C19 (CYP2C19) metabolizes arachidonic acid to produce epoxyicosanoid acids, which are involved in vascular tone and regulation of blood pressure. Recent findings suggest that CYP2C19 gene might be considered as a novel candidate gene for treatment of cardiovascular disease. The aim of the present study was to evaluate the association between two variants, CYP2C19*2 (681G>A) and CYP2C19*3 (636G>A) and the development of essential hypertension (EH) in Koreans.
Materials and Methods
We carried out an association study in a total of 1190 individuals (527 hypertensive subjects and 663 unrelated healthy controls). The CYP2C19 polymorphisms were genotyped using the SNaPShot™ assay.
Results
The distribution of alleles and genotypes of CYP2C19*3 showed significant difference between hypertensive patients and normal controls (p=0.011 and p=0.013, respectively). Logistic regression analysis indicated that the CYP2C19*3 (636A) allele carriers were significantly associated with EH [odds ratio, 0.691; 95% confidence interval (CI), 0.512-0.932, p=0.016], in comparison to wild type homozygotes (CYP2C19*1/*1). Neither genotype nor allele distribution of CYP2C19*2 polymorphism showed significant differences between hypertensive and control groups (p>0.05).
The pathogenesis of essential hypertension (EH) has not yet been clearly determined, nevertheless, it is known that a compound character is derived from a complex interaction of genetic and environmental factors. Various genes are responsible for the development of EH, but it is of a great interest to note a study of several different types of genes in the renin-angiotensin-aldosterone system:1 association studies between EH and genetic variants were carried worldwide, however, the relation between these two remains controversial due to different interpretations of the results depending on different analytic groups. Such differences in results are have been suggested to be due to diversity in genetic variations influenced by different age, gender, and ethnic backgrounds.2,3
The cytochrome P450, CYP, is a superfamily of cysteine-heme enzymes that regulate the oxidative metabolism of endogenous and exogenous molecules. The CYP enzymes are classified into families, sub-families and individual enzymes based on similarities in their amino acid sequence.4 There are 11 different types of the CYP family categorized in the human body.5 The CYP epoxygenase metabolizes arachidonic acid to epoxyeicosatrienoic acids (EETs), which are vasoactive and, affect renal sodium handling and water transport. In addition, these metabolites have been proposed to play a mechanistic role in the development of high blood pressure (BP).6 Genetic variants of CYP enzymes have been identified as one of the important reasons for interindividual, interethnic and interracial differences in drug response.7 Genetic polymorphisms in drug-metabolizing enzyme genes can cause enzyme variants with high, low, or no activity. Consequently, individuals can be classified into phenotypes of poor metabolizers (PM), intermediate metabolizers, extensive metabolizers (EM), or ultrarapid metabolizers.8
CYP2C exists mostly in the human liver, but the notable activation is known to be found in the walls of the digestive tracts. The CYP2Cs are an important subfamily of CYP enzymes that metabolize approximately 20% of most therapeutic drugs.9 The CYP2C subfamily that exist in the human body is composed of four types of isoenzymes, CYP2C8, CYP2C9, CYP2C18, and CYP2C19, and the genes that encode these enzymes are all located in the human chromosome 10q24.10 CYP2C19 is abundantly expressed in smooth muscle cells, artery endothelial and cardiac myocytes. It is also an important enzyme responsible for EETs synthesis. The potent effects of EETs on vascular tone and renal salt and water transport indicate that they might play a key role in BP regulation.11 CYP2C19 is highly polymorphic and can cause variability in drug response. There are 19 different types of variants reported, and seven of these variants encode an inactive enzyme including CYP2C19*2, *3, *4, *5, *6, *7 and *8 (http://www.imm.ki.se/CYPalleles). There are significant ethic differences in the distribution of these variants, with the PM phenotype found in 2-5% of Caucasians but in 13-23% of Asian populations.12 The most frequently found defective alleles in human populations are CYP2C19*2 (rs4244285), and they exist in two different variation types, CYP2C19*2A and CYP2C19*2B. CYP2C19*2 accounts for 75-85% of the alleles responsible for the PM phenotype in Caucasians and East Asians, which is transmitted as an autosomal dominant recessive pattern.12,13 CYP2C19*2 is a defective allele that eliminates the catalytic activity on all substrates, which carries a 681G<A single base substitute in exon 5 causing an alternate splice site consensus, thus dramatically lowering the drug metabolism.14 The CYP2C19*2 allele is significantly more frequent in East Asian populations (14-39%) than among Caucasians (8-16%) and Africans (18-25%).15-17 In Koreans, the frequency of CYP2C19*2 is reported to be 28%, similar to 27% in the Japanese population, but showing a large difference from the Chinese population.18
The second important PM allele is CYP2C19*3 (rs4986893), which has an adenine substituted for a guanine at base 636 (636G>A) in exon 4, creating a premature stop codon. At the time of discovery, CYP2C19*3 variant existed only in the Japanese group, and not found in the Caucasian group but only rarely.14 CYP2C19*3 is also found to be evenly distributed in East Asians; for instance, among the Chinese, Thai, Burmese, and Vietnamese populations.18,19 The frequency of CYP2C19*3 in the Korean population is reported to be 8%, similar to the Chinese (4.5%) and Vietnamese (5%) populations, but showed a great deal of difference from the Japanese (16%), Caucasians (0.9%) and Africans (0.6%).20,21
In recent association studies of CYP2C19 genetic polymorphism and cardiovascular disease (CVD) carried out on Caucasians, the group with CYP2C19*2/*2 homozygotes demonstrated a notable increase in the level of CVD and arteriosclerosis risk indicatiors,22 suggesting that CYP2C19*2 is the high risk factor related to CVD. Based on CYP2C19*2 and CYP2C19*3, individuals can be classified as PMs (CYP2C19*2/*2, *3/*3, and *2/*3) and EMs (CYP2C19*1/*1, *1/*2, and *1/*3). Consequently, CYP2C19 phenotypes can be predicted from the genotype combinations.23
Many studies on the CYP2C19 gene are focused mainly on the associations of genetic polymorphisms and the drug metabolism of many different classes of commonly used drugs. To our knowledge, however, no case-control study between genetic variants of the CYP2C19 gene and EH has been carried out. The purpose of this study was to assess the association between CYP2C19 genetic variants and EH in Koreans. Our findings on allele and genotype distribution patterns in Koreans will serve as a guideline in the standardization of personalized therapeutic tools.
Out of 2200 outpatients between the age of 20 and 65 who visited Yonsei University College of Medicine, Severance Hospital, Cardiovascular Center, Seoul, Republic of Korea, between November 2001 and February 2009 and control of people who received physical examination, we selected a study group of 1190 subjects. These consisted of 527 cases (265 males) suitable for the criteria of hypertension with sufficient clinical data and a control group of 663 (287 males), who consented to this study and to provide their blood samples. Blood pressure was measured after resting over 10 minutes, and was repeated 3 times at the interval of 5 minutes to acquire the mean blood pressure value. In this study, high blood pressure was defined as systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg before taking the medication and the patients with the history of taking anti-hypertensive drugs were included. The participants with prehypertension status (SBP=120-189 mm Hg, and/or DBP=80-89 mm Hg), and patients with secondary hypertension, kidney dysfunction, and diabetes were excluded from this study. The control group was selected from the normal men and women who had the measured SBP <120 mm Hg and DBP <80 mm Hg, with no history of hypertension, diabetes, kidney dysfunction, surgical procedures related to craniovascular diseases, and coronary artery disease or the family history of these diseases. Those who showed normal electrocardiogram readings and no clinical view of enlarged heart on the chest X-ray also selected. Medical records and the surveys to verify the age, gender, smoking and drinking habits, exercise and dietary habits, and past medical history of the participants were reviewed, while their height and weight were measured to calculate the body mass index (BMI) in kg/m2. After measuring the BP, blood samples were taken to measure the high-density lipoprotein cholesterol, triglyceride, fasting plasma glucose, and total cholesterol (TCHO). Low density lipoprotein cholesterol (LDL-C) values were calculated using the Friedewald equation.24 The haemotocrit was measured, and blood cells were frozen at -80℃ in the deep-freezer until experiment. All the participants submitted their written informed consent prior to the study. This study was approved by the local Institutional Review Board of Yonsei University College of Medicine, and was in accordance with the ethical guidelines of the Declaration of Helsinki.
Three milliliters of peripheral blood were taken with the ethylenediaminetetraacetic acid tube, and genomic DNA was extracted from the leukocyte nucleus by using the QuickGene SP kit DNA whole blood (Fujifilm Life Science, Tokyo, Japan) to analyses of the CYP2C19*2 and CYP2C19*3 polymorphisms. The DNA samples were quantitatively aliquoted to 25 ng/uL using Quant-iT double strand DNA BR Assay Kits (Invitrogen, Carlsbad, CA, USA), and each was inserted into a sample tube. A relatively simple analytic technique, TaqMan® Fluorogenic 5' Nuclease Assay (Applied Biosystems, Foster City, CA, USA), was used to determine CYP2C19*2 and CYP2C19*3 genotypes. The polymerase chain reaction (PCR) primer sequences used to determine the genotypes were prepared as described previously.25 The PCR was performed in a volume of 5 uL containing 10 ng of genomic DNA, 0.13 uL of 40X assay mix, and 2.5 uL of TaqMan® Universal PCU master mix. Real-time PCR was performed by using the ABI Prism 7900 HT Fast Real-Time PCR System (Applied Biosystems). The PCR cycling program was initiated with thermal pre-denaturation at 95℃ for 10 minutes, followed by 45 cycles of 95℃ for 15 seconds, 60℃ for 40 seconds, 72℃ for 1 minute, and a final extension at 72℃ for 10 minutes. After PCR reaction was over, the result was analysed by using the 7900 HT Sequence Detection System 2.3 software (Applied Biosystems).
Statistical analysis of the association analysis between the CYP2C19*2 and CYP2C19*3 variants and EH was done by using SPSS 14.0 for Windows (SPSS Inc., Chicago, IL, USA). All the statistical values were shown with mean±standard deviations. Student's t-test was used in the continuous variable differences in hypertensive patients and healthy control subjects, and the chi-square test or the Fisher's exact test was used for the comparison of the nominal variable as genotype frequency. The chi-square test was used to test the significance of the Hardy-Weinberg equilibrium (HWE) of the polymorphism of the two genes in the entire data of the patient and the control groups. For the measurement of relative risk of the genotypes in two groups, gender and age were adjusted, and the multiple regression analysis was performed. The partition-ligation algorithm was used to construct the individual haplotype profile, and the HAPLO program was used for the calculation of haplotype frequency and association analysis. The statistical level of significance for all the analyzed results was defined as the p-value to be less than 0.05.
The clinical characteristics of the study subjects are shown in Table 1. The male-female ratio of 50.47% in the patient group and 44.46% in the control group showed statistical difference. This is due to the difference in hypertensive conditions between the genders, and it can be explained by the fact that relatively large numbers of premenopausal women in the female control group are included. In both patient and control groups, all the other mean clinical values including SBP and DBP, except TCHO and LDL-C values, were different significantly from the hypertensive subjects and controls (p<0.001).
Distribution of the allele and genotype frequencies of hypertensive patients and normal control subjects, with regard to CYP2C19*2 and CYP2C19*3, are shown in Table 2. Genotype distributions of the two variants in the present study groups did not deviate significantly from HWE (p=0.916 and p=0.184, respectively), with very little selective bias between the two groups, and there was no genotyping error. No statistically significant differences in CYP2C19*2 (rs4244285, 681A) allele frequency were observed between hypertensive and control subjects (27.80% vs. 25.49%, p=0.234). Also, we found no difference in genotype frequency between hypertensive patients and controls (p=0.238). The CYP2C19*3 (rs4986893, 636A) defective allele is known to induce PM in the drug metabolism by producing abnormal enzymatic protein (W212X). CYP2C19*3 genotype distribution and allele frequency were significantly different between the hypertensive patient and control groups (p=0.013 and p=0.011, respectively).
Logistic regression analysis indicated that CYP2C19*3 was significantly associated with hypertension (Table 3). After adjustments for age and gender were made, the odds ratio (OR) of the CYP2C19*3 carriers (GA+AA) for the risk of hypertension over the wild type homozygotes (GG), was 0.691 [95% confidence interval (CI), 0.512-0.932, p=0.016] under a dominant mode of inheritance. Compared with CYP2C19*1/*1 homozygotes, the CYP2C19*3 allele was associated with a 30.9% reduced risk for the development of EH. In contrast, we did not find any significant ORs for hypertension which was related to any CYP2C19*2 genotypes (p>0.05). The prevalence of EH was not significantly elevated or reduced in individuals with the CYP2C19*2 (681A) carriers (p=0.450 in a dominant model) or 681AA genotype (p=0.161 in a recessive model).
EH is a complex trait disease in which genetic and environmental factors play a crucial role. A number of genes are thought to be responsible for its pathogenesis and numerous genes have been investigated for an association with EH, however, with controversial findings. In addition, there is no clear elucidation of how many genes are involved in the development of EH. Biochemical and pharmacological results from animal and human studies indicate that the CYP arachidonic acid pathway plays a mechanistic role in hypertension.26-28 Previous studies showed that several variants of CYP-related genes (CYP2C8, 2C9 and 2J2), were found to exhibit reduced functional activity in vitro and represent relatively common variants, and they provide an opportunity to examine the significance of this pathway in hypertensive cases. Recently, several studies on CYP2C19 genetic variants found significant association with cardiovascular diseases such as atherosclerosis29 and coronary artery disease,30 however, the role of CYP2C19 polymorphisms in hypertension or elevated BP has not sufficiently been examined. Ma, et al.31 found that hypertension is associated with the CYP2C19 rs10509676 variant homozygote (AA genotype) and rs10509676 A-rs11568732 G haplotype is an independent genetic risk factor for hypertension in a Han Chinese population.
Our present results demonstrated CYP2C19 genetic heterogeneity in Koreans, relative to other ethnic populations including Chinese, Japanese, Vietnamese, Thais, Caucasians and African-Americans. The frequency of the prevalent main defective allele of CYP2C19*2 in our Korean sample (26.5%) is similar to those of Thais (35.1%), Japanese (25.6%), Han Chinese (24.7%) and Vietnamese (23.6%), and was significant higher than that reported in Caucasians (11.2-16.3%) and Mexican-Americans (9.7%).17,22,32 Whereas, intermediated frequency was found in the Mongoloid population (18.2%).17 These results may indicate even sharing of the CYP2C19*2 allele among East Asian populations, and it can also be interpreted to suggest that they possess similarity in the genetic background for drug metabolism.
In this study, we observed that the frequency of the CYP2C19*3 allele had been regarded as an Asian-specific variant allele that accounted for the PM phenotype in Asian populations, which occurs rarely in Caucasians. The allele frequency of CYP2C19*3 in Koreans was 10.5%, and this frequency was similar to that of Japanese (10-15.6%), Mongolians (11.2%), Chinese Uygur (9.4%), and Chinese Kazakh (8.0%), however, relatively higher than those of Chinese Han (7.2%), Thais (5.0%), and Vietnamese (4.9%), and significantly higher than those of African-Americans (0.8%) and Caucasians (0.2%).15,16
To the best of our knowledge, we are the first to report potential
associations between functionally relevant CYP2C19*2 and *3 variants and EH in a relatively large Korean population (n=1190). Also, there are no such studies on any other ethnic groups for the comparison. These types of case-control studies have not so far been attempted, except for the analysis of change of pharmacological dynamic parameter of antihypertensive agents such as metoprolol (beta blocker). Our major finding in the present study was that Korean patients with EH had a lower frequency of the CYP2C19*3 variant allele than did normal controls. To verify the associations between the development or protection of hypertension and these variants of the CYP2C19 gene, multiple logistic regression analysis was performed under parameters adjusted for age and gender. Consequently, the CYP2C19*2 defective allele appeared to lack of association with hypertension. However, in the case of CYP2C19*3, the OR of 0.691 (95% CI, 0.512-0.932, p=0.016) under the dominant model (GA+AA/GG) was obtained proving that the CYP2C19*3 A allele carriers have a protective effect on the development of hypertension. Based on the variant allele of CYP2C19*3, individuals can be classified as PMs (CYP2C19*2/*2, *2/*3, *3/*3) and EMs (CYP2C19*1/*1, *1/*2, *1/*3).18 These results can be used as a reference to determine the adequate drug therapy on patients with different genotypes, when administering drugs of CYP2C19 substrate.
We have previously studied the association between CYP2C19 gene variants and clopidogrel resistance in Korean patients with coronary artery disease,33 and the result showed that the resistance to clopidogrel in subjects with the CYP2C19*3 A allele was 2.5-fold higher than that of subject who lacked this allele. This result proves that CYP2C19 is a risk-predictive gene by suppressing the anticoagulation reaction. Therefore, it is recommended to switch to other drugs or use subsidiary drugs additionally. In a recent study on the association between CYP2C19 variants and significant inflammation index related to the development of CVD done on Caucasians, it was shown the CYP2C19*2 defective allele has been shown to significantly increase the level of interleukin-6 and high sensitive C-reaction protein,22 indicating that the CYP2C19 gene is a major candidate gene for revealing the association study of development of CVD. Our present study suggests that the understanding of an association between CYP2C19 gene variants and hypertension that exists in a certain population will aid in tailoring healthcare to other populations, and provide an important genetic database for future personalized and predictive medicine. Therefore, an extensive study on various CYP2C19 gene variants, the occurrence of hypertension, and an association analysis of antihypertensive drugs with these results is warranted.
In conclusion, the case-control association study between the CYP2C19*2 and *3 variants and the development of hypertension was conducted in a relatively large number of Koreans for the first trial. The results showed that the frequency of CYP2C19*3 A allele which is uncommon in Caucasians, was found to be 10.5% in Koreans, showing a significant difference in frequency between hypertensive cases and normal controls. Furthermore, our results indicated that the CYP2C19*3 A allele was associated with EH in particular, and that this defective allele might be a protective effector against the development of hypertension. These results can clinically be used as a genetic reference in determining adequate drug therapy for hypertensive patients in the future.
Figures and Tables
Table 1
Baseline Clinical Characteristics of the Participants in Study Population
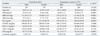
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; TG, triglyceride; TCHOL, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FPG, fasting plasma glucose.
Data values are means±standard deviation and numbers (%).
*p value was estimated by Student's t-test.
ACKNOWLEDGEMENTS
This study was supported by a grant of Korea Healthcare technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A000385), and Korea Government (MOEHRD, KRF-2005-206-C00017).
References
1. Aynacioglu AS, Sachse C, Bozkurt A, Kortunay S, Nacak M, Schröder T, et al. Low frequency of defective alleles of cytochrome P450 enzymes 2C19 and 2D6 in the Turkish population. Clin Pharmacol Ther. 1999. 66:185–192.

2. Bae Y, Park C, Han J, Hong YJ, Song HH, Shin ES, et al. Interaction between GNB3 C825T and ACE I/D polymorphisms in essential hypertension in Koreans. J Hum Hypertens. 2007. 21:159–166.

3. Turner ST, Boerwinkle E, Sing CF. Context-dependent associations of the ACE I/D polymorphism with blood pressure. Hypertension. 1999. 34(4 Pt 2):773–778.

4. Danielson PB. The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr Drug Metab. 2002. 3:561–597.

5. Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, et al. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996. 6:1–42.

6. Rahman M, Wright JT Jr, Douglas JG. The role of the cytochrome P450-dependent metabolites of arachidonic acid in blood pressure regulation and renal function: a review. Am J Hypertens. 1997. 10:356–365.

7. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev. 2003. 35:99–106.

8. Chen L, Qin S, Xie J, Tang J, Yang L, Shen W, et al. Genetic polymorphism analysis of CYP2C19 in Chinese Han populations from different geographic areas of mainland China. Pharmacogenomics. 2008. 9:691–702.

9. Goldstein JA. Clinical relevance of genetic polymorphisms in the human CYP2C subfamily. Br J Clin Pharmacol. 2001. 52:349–355.

10. Gray IC, Nobile C, Muresu R, Ford S, Spurr NK. A 2.4-megabase physical map spanning the CYP2C gene cluster on chromosome 10q24. Genomics. 1995. 28:328–332.

11. Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002. 82:131–185.

12. de Morais SM, Goldstein JA, Xie HG, Huang SL, Lu YQ, Xia H, et al. Genetic analysis of the S-mephenytoin polymorphism in a Chinese population. Clin Pharmacol Ther. 1995. 58:404–411.

13. Brøsen K, de Morais SM, Meyer UA, Goldstein JA. A multifamily study on the relationship between CYP2C19 genotype and s-mephenytoin oxidation phenotype. Pharmacogenetics. 1995. 5:312–317.

14. De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994. 46:594–598.
15. Goldstein JA, Ishizaki T, Chiba K, de Morais SM, Bell D, Krahn PM, et al. Frequencies of the defective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Pharmacogenetics. 1997. 7:59–64.

16. Ozawa S, Soyama A, Saeki M, Fukushima-Uesaka H, Itoda M, Koyano S, et al. Ethnic differences in genetic polymorphisms of CYP2D6, CYP2C19, CYP3As and MDR1/ABCB1. Drug Metab Pharmacokinet. 2004. 19:83–95.

17. Luo HR, Poland RE, Lin KM, Wan YJ. Genetic polymorphism of cytochrome P450 2C19 in Mexican Americans: a cross-ethnic comparative study. Clin Pharmacol Ther. 2006. 80:33–40.

18. Yamada S, Onda M, Kato S, Matsuda N, Matsuhisa T, Yamada N, et al. Genetic differences in CYP2C19 single nucleotide polymorphisms among four Asian populations. J Gastroenterol. 2001. 36:669–672.

19. Tassaneeyakul W, Mahatthanatrakul W, Niwatananun K, Na-Bangchang K, Tawalee A, Krikreangsak N, et al. CYP2C19 genetic polymorphism in Thai, Burmese and Karen populations. Drug Metab Pharmacokinet. 2006. 21:286–290.

20. Herrlin K, Massele AY, Jande M, Alm C, Tybring G, Abdi YA, et al. Bantu Tanzanians have a decreased capacity to metabolize omeprazole and mephenytoin in relation to their CYP2C19 genotype. Clin Pharmacol Ther. 1998. 64:391–401.

21. Lee SS, Lee SJ, Gwak J, Jung HJ, Thi-Le H, Song IS, et al. Comparisons of CYP2C19 genetic polymorphisms between Korean and Vietnamese populations. Ther Drug Monit. 2007. 29:455–459.

22. Bertrand-Thiébault C, Berrahmoune H, Thompson A, Marie B, Droesch S, Siest G, et al. Genetic Polymorphism of CYP2C19 gene in the Stanislas cohort. A link with inflammation. Ann Hum Genet. 2008. 72(Pt 2):178–183.
23. Mrazek DA, Biernacka JM, O'Kane DJ, Black JL, Cunningham JM, Drews MS, et al. CYP2C19 variation and citalopram response. Pharmacogenet Genomics. 2011. 21:1–9.

24. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972. 18:499–502.

25. Zhou Q, Yu XM, Lin HB, Wang L, Yun QZ, Hu SN, et al. Genetic polymorphism, linkage disequilibrium, haplotype structure and novel allele analysis of CYP2C19 and CYP2D6 in Han Chinese. Pharmacogenomics J. 2009. 9:380–394.

26. Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000. 275:40504–40510.

27. Laffer CL, Laniado-Schwartzman M, Wang MH, Nasjletti A, Elijovich F. Differential regulation of natriuresis by 20-hydroxyeicosatetraenoic Acid in human salt-sensitive versus salt-resistant hypertension. Circulation. 2003. 107:574–578.

28. Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with angiotensin salt-sensitive hypertension. Hypertension. 2003. 41(3 Pt 2):709–714.

29. Ercan B, Ayaz L, Ciçek D, Tamer L. Role of CYP2C9 and CYP2C19 polymorphisms in patients with atherosclerosis. Cell Biochem Funct. 2008. 26:309–313.

30. Yang YN, Wang XL, Ma YT, Xie X, Fu ZY, Li XM, et al. Association of interaction between smoking and CYP 2C19*3 polymorphism with coronary artery disease in a Uighur population. Clin Appl Thromb Hemost. 2010. 16:579–583.

31. Ma Y, Ni W, Zhu W, Xiong Y, Deng X. Association of genetic polymorphisms of CYP 2C19 with hypertension in a Chinese Han population. Blood Press. 2011. 20:166–170.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download