Abstract
Stem cells are emerging as therapeutic candidates in a variety of diseases because of their multipotent capacities. Among these, mesenchymal stem cells (MSCs) derived from bone marrow, umbilical cord blood or adipose tissue, comprise a population of cells that exhibit extensive proliferative potential and retain the ability to differentiate into multiple tissue-specific lineage cells including osteoblasts, chondrocytes, and adipocytes. MSCs have also been shown to enhance neurological recovery, although the therapeutic effects seem to be derived from an indirect paracrine effect rather than direct cell replacement. MSCs secrete neurotrophic factors, promote endogenous neurogenesis and angiogenesis, encourage synaptic connection and remyelination of damaged axons, decrease apoptosis, and regulate inflammation primarily through paracrine actions. Accordingly, MSCs may prevail as a promising cell source for cell-based therapy in neurological diseases.
Stem cells are emerging as therapeutic candidates in a variety of disease because of their multipotent capacities. Embryonic stem cells are pluripotent and can differentiate into all specialized cell types derived from the three embryonic germ layers. Nevertheless, both ethical and technical considerations limit the clinical availability of these cells. Other potential cell sources, especially for central nervous system (CNS) repair, include fetal neural stem cells (NSCs) and neural precursor cells (NPCs). NSCs can be expanded over multiple passages and do not require the recapitulation of early developmental signals to induce neuroectodermal commitment, as they are already neuralized and committed to a CNS cell fate.1 However, transplantation of fetal NSCs into the adult brain encompasses numerous ethical and scientific hurdles.1-3 Because of their plastic ability to survive as undifferentiated cells in ectopic perivascular niches,4 NPCs have been tested in animal models of neurological diseases. These cells also release paracrine factors that foster survival and proliferation of endogenous neural progenitor cells. However, the application of terminal differentiation of NPCs into neural-lineage cells to replace damaged cells remains controversial.5
In contrast, adult stem cells, responsible for maintaining the homeostasis of a specific tissue with fewer ethical problems. One of the most extensively studied populations of multipotent adult stem cells is mesenchymal stem cells (MSCs), which are derived from bone marrow (BM). They can also be isolated from other tissues such as umbilical cord blood,6 synovium,7 periosteum,8 peripheral blood,9 adipose tissue,10 skeletal muscle,11 and placental tissue.12 MSCs are an excellent candidate for cell therapy because they are easily accessible; can be easily isolated and expanded rapidly in vitro;13 are multipotent;14,15 involve minimal loss of potency;16,17 form supportive stroma for hematopoiesis and support stem cell engraftment;18 may not require immune suppression;19,20 seem to be largely immunologically inert, paving the way for allogeneic transplantation;21 secrete numerous trophic factors that modulate inflammation and apoptosis.22 The large body of work that has accumulated since the discovery of human MSCs has convincingly shown that MSCs from diverse sources retain the ability to differentiate into the mesodermal lineage cells including osteoblasts, chondrocytes and adipocytes.23,24 They also exhibit the ability to differentiate into neurons-like cells,25 myocytes and skeletal muscle,23 although there is a lack of definitive evidence as to the functionality of these differentiated cells.26,27 Nevertheless, MSCs have considerably contributed to tissue repair in myocardial infarction (MI),28 stroke,29,30 meniscus injury,31 and limb ischemia.32
In this review, we will discuss the therapeutic mechanisms of MSCs for neurorestoration and neural regeneration. Thereafter, we will review the published reports of clinical trials for a variety of neurological diseases including stroke, traumatic brain injury, spinal cord injury, Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS).
MSCs secrete a variety of cytokines and growth factors that promote endogenous neuronal growth, neurogenesis and angiogenesis, encourage synaptic connection and remyelination of damaged axons, decrease apoptosis, and regulate inflammation primarily through paracrine actions (Fig. 1). Human MSCs are known to secrete neurotrophic factors including brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor, glial cell line-derived neurotrophic factor (GDNF), and nerve growth factor (NGF). After direct transplantation in an animal model of stroke, human MSCs were shown to integrate into host brain, survive, differentiate into neurons and astrocytes, and induce neurobehavioral improvement.33 BM-derived MSCs can secrete various trophic factors, the secretion of which is enhanced under post-ischemic conditions.34,35 In our previous study using a rat model of spinal cord injury, neurally induced cells derived from umbilical cord blood also exhibited better functional recovery in vivo and secreted more NGFs in vitro.36
MSCs induce the proliferation of endogenous neural stem/progenitor cells in the subventricular zone (SVZ) and are critical to the survival of newborn cells.37 They have been shown to be directly involved in neural differentiation after engraftment into damaged tissue and migrate to the CNS to a limited extent.38 Of particular note, genetically modified MSCs expressing Neurogenin1, a proneuronal gene that directs neural differentiation, increased the therapeutic effects of MSCs in ischemic brain.39 In addition, MSCs promote the plasticity of damaged neurons and activate astroglial cells to secrete neurotrophins such as BDNF, GDNF and NGF.34 In an animal model of stroke, intravenous transplantation of BM stromal cells improved functional outcomes by promoting endogenous repair.29
Previous study suggested that extracellular matrix components derived from MSCs can enhance nervous system repair.40 For example, fibronectin prominently performs essential roles in neuronal survival, axonal sprouting and synaptogenesis following cerebral ischemia.41 Moreover, extracellular matrix molecules and cell adhesion molecules such as integrin, cadherin, and selectin can promote axonal growth and regeneration.42
Reportedly intravenous transplantation of MSCs reduced apoptotic cells stained with terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling in an animal model of middle cerebral artery occlusion.29 This anti-apoptotic effect, together with the previously described capacity to release neurotrophic molecules, may well explain the remarkable functional recovery obtained with the administration of MSCs in experimental models of stroke as well as spinal cord injury.43,44
MSCs were shown to exert immunomodulatory properties in vitro.45 These features were exploited by researchers in the treatment models of MS and experimental autoimmune encephalomyelitis.46,47 In PD, MSCs act as neuroprotectors via an anti-inflammatory response to regulate the activity of microglia and to protect dopaminergic neurons.48,49 In ischemic brain, MSCs were also useful as an immunomodulator.50 MSCs reduced the numbers of Iba-1+ and ED1+ inflammatory cells.39 In our previous study, intravenously transplanted MSCs did not only decrease the level of pro-inflammatory cytokine IL-1β and the proportion of activated microglia, but also increased the level of anti-inflammatory cytokine IL-10, potentially suggesting that early immunomodulation by MSCs was an underlying mechanism of functional recovery in spinal cord injured rats.51
MSCs secrete a number of growth factors and cytokines, which normally support the proliferation of hematopoietic stem/progenitor cells.22 In experimental models of cardiovascular diseases such as MI and limb ischemia, the secretion of multiple angiogenic cytokines such as hepatocyte growth factor, basic fibroblast growth factor, insulin-like growth factor 1 and vascular endothelial growth factor was induced from MSCs.52-56 Existing evidence suggests that these cells can ameliorate ischemic tissue injury, produce appropriate cytokine milieu to promote angiogenesis, and possibly differentiate into endothelial cells.54,55,57 The evidence seems to point toward the theory that a complex set of trophic factors secreted by MSCs significantly contributes to injury repair in vivo by stimulating angiogenesis.
MSCs are believed to secrete neurotrophic factors, immunomodulatory cytokines, pro-angiogenic factors, extracellular matrix molecules and so on. Thus, the therapeutic effects of transplanted MSCs seem to be derived from paracrine effects rather than direct cell replacement. It is conceivable that MSCs or host brain cells stimulated by grafts may produce such proteins to induce functional recovery and reorganization.58 MSC-induced secretion of beneficial cytokines by host cells in subjects with neurological diseases such as stroke29 as well as spinal cord injury33,44,59 would be a more plausible mechanism given that activation of recipient host cells after cell transplantation has been previously described in other disease models such as MI and ischemic vascular disease.60,61
Several clinical reports on MSC-based treatments have been published in the past decade, and evoked great excitement for therapeutic candidates for several diseases.62 As early as the 1990s, cultured MSCs have already been supplemented to reduce acute and chronic graft-versus-host disease among patients receiving allogenic hematopoietic stem cell transplantation, as well as to ameliorate clinical symptoms in osteogenesis imperfecta and glycogen storage disease.62-64 Currently, the effective therapeutic benefits of MSCs have been supported by increasing numbers of clinical trials on various disorders such as MI, cancer, diabetes mellitus, and Crohn's disease.65-68
We will discuss the role of MSCs in neurological diseases, spanning the clinical trials on stroke, spinal cord injury, PD, ALS, and MS. A summary of the cell source, route of delivery, number of patients, study design, dose of transplant, outcome measures and main results for each therapeutic application of MSCs is provided in Table 1.
Neuronal and astroglial damages can occur in cerebrovascular disease resulting from the blockage of blood flow in selected brain areas, leading to motor, sensory and cognitive dysfunctions.3 It has been shown that stroke-induced endogenous neurogenesis and migration of neural stem or progenitor cells into regions of ischemic damage occurs in humans, but the extent to which neurogenesis is able to replace lost neurons or contributes to functional improvement in stroke patients is largely limited.3,69,70 The limited therapeutic efficacy of endogenous repair processes has encouraged clinicians to incorporate MSCs or BM-derived cells in restorative strategies.
Clinical trials for MSC transplantation to treat stroke and traumatic brain injury are currently ongoing. In patients with middle cerebral artery infarction, the use of autologous MSCs derived from BM has indicated no safety concerns for death, stroke recurrence, or serious adverse events up to 1 year, and trends towards increased functional recovery.71 This group also reported as a long-term follow-up study for 5 years no serious adverse effects following MSC treatment.72 Direct administration of MSCs to an injured region following traumatic brain injury has also been performed without adverse events. Briefly, seven patients each received up to 109 expanded MSCs as part of a cranial repair operation. The patients were followed up for six months and demonstrated significant improvements in neurological function.73,74
Depending on the severity and location of injury, patients present with a varying range of functional impairments, arising from both damage to the local circuitry of the spinal cord and disruption of the ascending and descending fiber tracts.75,76 All groups who have tested the safety of the transplantation of BM-derived mononuclear cells and stromal cells, or adipose tissue-derived MSCs in patients with spinal cord injury indicate that administration of these cells does not cause any serious adverse effects.77-80 Geffner, et al.77 investigated the improvement in quality of life and bladder function without pain or tumor up to 2 years. Syková, et al.78 reported that five patients who received cells intra-arterially showed improvement up to 1 year.
PD is a progressive neurodegenerative disease whose dopaminergic neurons selectively degenerate in the substantia nigra. Although a variety of drugs such as L-dopa are available, they only remain effective for a certain period in most patients. The limitation of pharmacologic agents increases the need for cell-based therapy as a restorative strategy. In a study recently reported by Li, et al.,81 two subjects with PD who underwent transplantation of fetal mesencephalic dopaminergic neurons, which had survived for over 10 years, but later developed α-synuclein-positive Lewy bodies in the engrafted donor neurons, suggesting that the disease can propagate from host to graft cells. On the other hand, when autologous BM-derived MSCs were transplanted into the SVZ by stereotaxic surgery, the results suggested the treatment to be safe, and no serious adverse events occurred after transplantation in PD.82 Additionally, when patients with multiple system atrophy (MSA) were treated with MSCs, greater improvement was noted on the unified MSA rating scale than in untreated control patients, and no delayed adverse effects related to MSC infusion occurred during the 12-month study period.83
ALS involves a pathology that causes a selective loss of motor neurons leading to a progressive decline in muscle function and poor prognosis. When Nagano, et al. completed a small double-blind clinical trial to assess the effect of intrathecal administration of IGF-1 on disease progression in nine patients with ALS, the high-dose treatment slowed the decline of motor functions, but not bulbar function or vital capacity.84 On the other hand, both intravenous and intrathecal administration of autologous MSCs were well tolerated, with some preliminary evidence of efficacy in patients with ALS.85-87 However, large controlled clinical studies are needed to assess possibility for this therapeutic strategy.
Most phase 1 studies for the safety of MSCs have been conducted in MS.85,88,89 As a result, Mohyeddin, et al.88 reported iatrogenic meningitis and headache; Yamout, et al.89 reported transient encephalopathy and seizure; and Karussis, et al.85 reported fever, headache and aseptic meningitis. Although serious adverse events related with cell transplantation are likely to be extremely uncommon in MS, the therapeutic efficacy in regards to clinical improvement remains controversial.85,88,89
Experimental evidence in preclinical models of neurological diseases suggests that MSCs are a promising candidate for achieving neural repair and protection. However, current data do not support the possibility that most of the reported effects occur as a result of direct cell replacement. Instead, indirect paracrine mechanisms, including a potent anti-inflammatory capacity, the release of anti-apoptotic and neurotrophic factors, and the ability to induce proliferation of local neural stem/progenitor cells, are likely to promote neurorestoration. Despite tremendous advancements, major unresolved issues concerning therapeutic application still exist. Especially, transplanted MSCs suffer from poor survival and engraftment into host tissue. Further studies are necessary to evaluate in depth the efficacy and safety of MSC-based therapy and whether such treatment would involve a high benefit-to-risk ratio in neurological diseases.
Figures and Tables
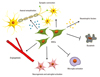 | Fig. 1Potential therapeutic mechanisms of neurorestoration using mesenchymal stem cells. MSCs secrete a variety of neurotrophic factors that promote endogenous neuronal growth, induce angiogenesis, neurogenesis and astroglial activation, encourage synaptic connection and axonal remyelination, decrease apoptosis, and regulate microglial activation primarily through paracrine actions. MSCs, mesenchymal stem cells. |
ACKNOWLEDGEMENTS
This study was supported by grants from National Research Foundation (NRF-2010-0020408; 2010-0024334), Yonsei University College of Medicine (6-2011-0078), and Stem Cell Research Center of the 21st Century Frontier Research Program (SC-4160) funded by the Ministry of Science and Technology, Republic of Korea.
References
1. Gogel S, Gubernator M, Minger SL. Progress and prospects: stem cells and neurological diseases. Gene Ther. 2011. 18:1–6.

2. Lovell-Badge R. The regulation of human embryo and stem-cell research in the United Kingdom. Nat Rev Mol Cell Biol. 2008. 9:998–1003.

3. Mathews DJ, Sugarman J, Bok H, Blass DM, Coyle JT, Duggan P, et al. Cell-based interventions for neurologic conditions: ethical challenges for early human trials. Neurology. 2008. 71:288–293.

4. Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011. 10:649–656.

5. Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006. 7:395–406.

6. Erices AA, Allers CI, Conget PA, Rojas CV, Minguell JJ. Human cord blood-derived mesenchymal stem cells home and survive in the marrow of immunodeficient mice after systemic infusion. Cell Transplant. 2003. 12:555–561.

7. De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001. 44:1928–1942.

8. Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, et al. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 2003. 11:55–64.

9. Villaron EM, Almeida J, López-Holgado N, Alcoceba M, Sánchez-Abarca LI, Sanchez-Guijo FM, et al. Mesenchymal stem cells are present in peripheral blood and can engraft after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004. 89:1421–1427.
10. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001. 7:211–228.

11. Cao B, Zheng B, Jankowski RJ, Kimura S, Ikezawa M, Deasy B, et al. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003. 5:640–646.

12. Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008. 26:300–311.

13. Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002. 20:530–541.

14. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.

15. Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007. 25:2896–2902.

16. Lee MW, Yang MS, Park JS, Kim HC, Kim YJ, Choi J. Isolation of mesenchymal stem cells from cryopreserved human umbilical cord blood. Int J Hematol. 2005. 81:126–130.

17. Kotobuki N, Hirose M, Takakura Y, Ohgushi H. Cultured autologous human cells for hard tissue regeneration: preparation and characterization of mesenchymal stem cells from bone marrow. Artif Organs. 2004. 28:33–39.

18. Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006. 20:161–171.

19. Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003. 76:1208–1213.

20. DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators Inflamm. 2010. 2010:865601.

21. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003. 31:890–896.

22. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006. 98:1076–1084.

23. Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009. 1176:101–117.

24. Vemuri MC, Chase LG, Rao MS. Mesenchymal stem cell assays and applications. Methods Mol Biol. 2011. 698:3–8.

25. Bae KS, Park JB, Kim HS, Kim DS, Park DJ, Kang SJ. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med J. 2011. 52:401–412.

26. Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005. 7:393–395.

27. Bianco P, Kuznetsov SA, Riminucci M, Gehron Robey P. Postnatal skeletal stem cells. Methods Enzymol. 2006. 419:117–148.

29. Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003. 73:778–786.

30. Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005. 49:407–417.

31. Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003. 48:3464–3474.

32. Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008. 26:2173–2182.

33. Nagai A, Kim WK, Lee HJ, Jeong HS, Kim KS, Hong SH, et al. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS One. 2007. 2:e1272.

34. Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002. 59:514–523.

35. Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z, et al. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007. 27:355–363.

36. Cho SR, Yang MS, Yim SH, Park JH, Lee JE, Eom YW, et al. Neurally induced umbilical cord blood cells modestly repair injured spinal cords. Neuroreport. 2008. 19:1259–1263.

37. Yoo SW, Kim SS, Lee SY, Lee HS, Kim HS, Lee YD, et al. Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp Mol Med. 2008. 40:387–397.

38. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999. 96:10711–10716.

39. Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, et al. Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells. 2008. 26:2217–2228.

40. Maltman DJ, Hardy SA, Przyborski SA. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem Int. 2011. 59:347–356.

41. Sakai T, Johnson KJ, Murozono M, Sakai K, Magnuson MA, Wieloch T, et al. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med. 2001. 7:324–330.

42. Giger RJ, Hollis ER 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2010. 2:a001867.

43. Akiyama Y, Radtke C, Honmou O, Kocsis JD. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002. 39:229–236.

44. Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine (Phila Pa 1976). 2004. 29:1971–1979.

45. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008. 8:726–736.

46. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005. 106:1755–1761.

47. Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007. 61:219–227.

48. Kim YJ, Park HJ, Lee G, Bang OY, Ahn YH, Joe E, et al. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009. 57:13–23.

49. Chao YX, He BP, Tay SS. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson's disease. J Neuroimmunol. 2009. 216:39–50.

50. Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008. 105:14638–14643.

51. Seo JH, Jang IK, Kim HB, Yang MS, Lee JE, Kim HE, et al. Immunomodulation from intravenous transplantation of mesenchymal stem cells promotes functional recovery in spinal cord injured rats. Cell Med. 2011. 2:55–67.

52. Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006. 20:661–669.

53. Cai L, Johnstone BH, Cook TG, Liang Z, Traktuev D, Cornetta K, et al. Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells. 2007. 25:3234–3243.

54. Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005. 25:2542–2547.

55. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004. 109:1292–1298.

56. Sadat S, Gehmert S, Song YH, Yen Y, Bai X, Gaiser S, et al. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem Biophys Res Commun. 2007. 363:674–679.

57. Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R, Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004. 110:349–355.

58. Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006. 198:54–64.

59. Cho SR, Kim YR, Kang HS, Yim SH, Park CI, Min YH, et al. Functional recovery after the transplantation of neurally differentiated mesenchymal stem cells derived from bone barrow in a rat model of spinal cord injury. Cell Transplant. 2009. 18:1359–1368.

60. Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, et al. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007. 204:3257–3269.

61. Tateno K, Minamino T, Toko H, Akazawa H, Shimizu N, Takeda S, et al. Critical roles of muscle-secreted angiogenic factors in therapeutic neovascularization. Circ Res. 2006. 98:1194–1202.

62. Si YL, Zhao YL, Hao HJ, Fu XB, Han WD. MSCs: biological characteristics, clinical applications and their outstanding concerns. Ageing Res Rev. 2011. 10:93–103.

63. Koc ON, Lazarus HM. Mesenchymal stem cells: heading into the clinic. Bone Marrow Transplant. 2001. 27:235–239.

64. Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002. 99:8932–8937.

65. Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005. 115:572–583.

66. Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009. 69:4134–4142.

67. Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010. 6:139–148.

68. Pistoia V, Raffaghello L. Potential of mesenchymal stem cells for the therapy of autoimmune diseases. Expert Rev Clin Immunol. 2010. 6:211–218.

69. Minger SL, Ekonomou A, Carta EM, Chinoy A, Perry RH, Ballard CG. Endogenous neurogenesis in the human brain following cerebral infarction. Regen Med. 2007. 2:69–74.

70. Ekonomou A, Ballard CG, Pathmanaban ON, Perry RH, Perry EK, Kalaria RN, et al. Increased neural progenitors in vascular dementia. Neurobiol Aging. 2011. 32:2152–2161.

71. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005. 57:874–882.

72. Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. STARTING collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010. 28:1099–1106.

73. Zhang ZX, Guan LX, Zhang K, Zhang Q, Dai LJ. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury. Cytotherapy. 2008. 10:134–139.

74. Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010. 5:933–946.

76. Sahni V, Kessler JA. Stem cell therapies for spinal cord injury. Nat Rev Neurol. 2010. 6:363–372.

77. Geffner LF, Santacruz P, Izurieta M, Flor L, Maldonado B, Auad AH, et al. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: comprehensive case studies. Cell Transplant. 2008. 17:1277–1293.

78. Syková E, Homola A, Mazanec R, Lachmann H, Konrádová SL, Kobylka P, et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006. 15:675–687.

79. Pal R, Venkataramana NK, Bansal A, Balaraju S, Jan M, Chandra R, et al. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy. 2009. 11:897–911.

80. Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011. 20:1297–1308.

81. Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008. 14:501–503.

82. Venkataramana NK, Kumar SK, Balaraju S, Radhakrishnan RC, Bansal A, Dixit A, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson's disease. Transl Res. 2010. 155:62–70.

83. Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008. 83:723–730.

84. Nagano I, Shiote M, Murakami T, Kamada H, Hamakawa Y, Matsubara E, et al. Beneficial effects of intrathecal IGF-1 administration in patients with amyotrophic lateral sclerosis. Neurol Res. 2005. 27:768–772.

85. Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010. 67:1187–1194.

86. Mazzini L, Mareschi K, Ferrero I, Vassallo E, Oliveri G, Nasuelli N, et al. Stem cell treatment in Amyotrophic Lateral Sclerosis. J Neurol Sci. 2008. 265:78–83.

87. Mazzini L, Ferrero I, Luparello V, Rustichelli D, Gunetti M, Mareschi K, et al. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: A Phase I clinical trial. Exp Neurol. 2010. 223:229–237.

88. Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, Alimoghaddom K, Talebian F, Hooshmand F, et al. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol. 2007. 4:50–57.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download