Abstract
Bronchiolitis obliterans (BO) is a late onset complication of allogeneic hematopoietic stem cell transplantation (HSCT), and treatment outcome is dismal if it does not respond to immunosuppressive therapy. A 21-year-old male diagnosed with acute myeloid leukemia received an allogeneic HSCT from human leukocyte antigen- identical sibling donor. Twenty one months after transplantation, he developed progressive dyspnea and was diagnosed BO. Despite standard immunosuppressive therapy, the patient rapidly progressed to respiratory failure and Novalung® interventional lung-assist membrane ventilator was applied in the intensive care unit. Three months after the diagnosis of BO, the patient underwent bilateral lung transplantation (LT) and was eventually able to wean from the ventilator and the Novalung®. Since the LT, the patient has been under a strict rehabilitation program in order to overcome a severe lower extremity weakness and muscle atrophy. Histologic findings of the explanted lungs confirmed the diagnosis of BO. Nine months after the LT, the patient showed no signs of rejection or infectious complications, but still required rehabilitation treatment. This is the first LT performed in a patient with BO after allogeneic HSCT in Korea. LT can be an effective therapy in terms of survival for patients with respiratory failure secondary to development of BO following HSCT.
Allogeneic hematopoietic stem cell transplantation (HSCT) is a curative treatment for many hematologic malignancies including acute myeloid leukemia (AML).1 However, chronic graft-versus-host disease (GVHD) is a major complication and a main cause of late mortality following allogeneic HSCT.2-4 Bronchiolitis obliterans (BO) is a late onset noninfectious pulmonary complication occurring in 10% to 15% of patients with extensive chronic GVHD.3 The mortality rate of BO after HSCT is very high, although BO is treated with high-dose systemic corticosteroid and immunosuppressive agents as in chronic GVHD. Furthermore, to our best knowledge, there are no controlled trials regarding the treatment of BO. The role of immunosuppressive agents is marginal in BO and many other therapeutic strategies have been reported. There are a few reports of lung transplantation (LT) in BO with encouraging results,5 and we report a first successful case of LT for BO following allogeneic HSCT in Korea.
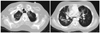
A 21-year-old male diagnosed as AML received an allogeneic HSCT from his human leukocyte antigen-identical sibling donor. Twenty one months after the transplantation, the patient was admitted due to progressive dyspnea, and computerized tomography examination of the chest showed diffuse bronchiectasis and air-trapping in both lungs with peribronchial ground glass opacity (Fig. 1), which was compatible with BO combined with pneumonia. Sputum culture showed the growth of pseudomonas aeruginosa, while bronchial washing culture showed the growth of Staphylococcus aureus (methicillin-resistant Staphylococcus aureus). Pulmonary function test showed evidence of BO with reduction in forced expiratory volume in one second (25% of expected) and diffusion capacity (48% of expected). Despite standard immunosuppressive therapy consisting of steroid, FK-506, and mycophenolate mofetil, the symptoms aggravated, and rapidly progressed to respiratory failure and became completely dependent on oxygen supply via mechanical ventilator. Hypercapnea could not be managed even with mechanical ventilator support, and therefore, Novalung® interventional lung-assist membrane was applied. There was no other therapeutic option except lung transplantation (LT) in order to treat end-stage BO, therefore, he was transferred to our hospital. Three months after the onset of BO and one month after the Novalung® application, he underwent bilateral LT from unrelated donor. Post-transplant course for LT was uneventful, and the patient was able to wean from the Novalung® on postoperative day (POD) 1 day and from the ventilator on POD 16 days. Histologic examination of the explanted lung showed obliterated bronchiole, patch interstitial fibrosis and intra-alveolar collection of histiocytes, which was compatible with BO (Fig. 2). He recovered from pulmonary edema and pneumonia, and was transferred to general ward on POD 20 days without necessitating oxygen support. Since the lung transplantation, the patient has been taking immunosuppressive agents including FK-506, mycophenolate mofetil and corticosteroids adjusted according to trough levels. Postoperative antimicrobial prophylaxis consisted of broad-spectrum antibacterial agents, gancyclovir and itraconazole. Currently, at 38 months after allogeneic HSCT and 10 months after LT, the patient still maintains complete chimerism without sign of respiratory failure or graft rejection.
The incidence of BO following HSCT is reported as 1.7-32%.6,7 It is a progressive, insidious lung disease occurring in patients after allogeneic HSCT, although the pathogenesis of BO following allogeneic HSCT remains not well defined. The standard diagnosis is the pathologic confirmation through lung biopsy. However, due to its invasiveness for the patients with chronic GVHD and BO, the National Institutes of Health provided that BO can be clinically diagnosed via pulmonary function test, radiologic testing in the absence of respiratory tract infection.2 International Bone Marrow Transplant Registry data suggest peripheral blood stem cell source, long duration to transplant, female donor to male recipient, prior episode of interstitial pneumonitis in addition to acute GVHD, and busulfan-based conditioning regimen as risk factors.6 However, there is no consistent risk factor except the presence of chronic GVHD.7
There is no standard treatment for BO. In general, the management of BO consists of immunosuppressive agents such as high dose corticosteroid, or calcineurin inhibitor such as cyclosporine A or tacrolimus. If a patient does not respond to conventional immunosuppressive treatment, the 2-year and 5-year survival rates are 20% and 13%, respectively.8 Recently, other therapeutic approach such as imatinib mesylate, antithymocyte globulins, anti-tumor necrosis factor-α and extracorporeal photodynamic therapy have been tried for treatment of BO.9-12 However, the efficacy and long-term results of adding such methods to the treatment for BO following HSCT is not yet known. There are a few reports of LT for BO following HSCT since Calhoon, et al.5 first reported a case in a 25 year old woman with acute lymphoblastic leukemia and restrictive lung disease treated with single LT following HSCT.3,13-15 Recently, Oshima, et al.16 reported a living-donor lobar lung transplantation for therapy-resistant BO after allogenetic HSCT from the same donor of bone marrow. In general, the donor of LT is different from hematopoietic stem cell donor. Therefore, there is high risk of rejection and may develop BO event in the transplanted lung. It has been reported that allogeneic HSCT would increase the risk of rejection after LT because of the amount of immunocompetent leukocytes present in the donor lung. However, most of LT do not experience graft rejection because long term immunosuppresion prior to LT might have induced a down-regulation of alloreactivity.5,13,17 In our case, the patient did not experience graft rejection.
Herein, we report the first case of LT for BO after allogeneic HSCT in Korea. Prior to LT, the patient was completely dependent on mechanical ventilator and rapidly progressed to respiratory failure. After LT, the patient underwent rehabilitation without any sign of respiratory failure or graft rejection, and needed special attention regarding the infectious complications or post transplant lymphoproliferative disorders. LT could be an effective therapy in terms of quality of life and survival for patients with respiratory failure secondary to the development of BO after allogeneic HSCT.
Figures and Tables
References
2. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005. 11:945–956.

3. Redel-Montero J, Bujalance-Cabrera C, Vaquero-Barrios JM, Santos-Luna F, Arenas-De Larriva M, Moreno-Casado P, et al. Lung transplantation for bronchiolitis obliterans after allogenic bone marrow transplantation. Transplant Proc. 2010. 42:3023–3025.

4. Lee JW, Lee DH, Jang PS, Yi MS, Chung NG, Cho B, et al. Prognostic implications of the NIH consensus criteria in children with chronic graft-versus-host disease. Yonsei Med J. 2011. 52:779–786.

5. Calhoon JH, Levine S, Anzueto A, Bryan CL, Trinkle JK. Lung transplantation in a patient with a prior bone marrow transplant. Chest. 1992. 102:948.

6. Santo Tomas LH, Loberiza FR Jr, Klein JP, Layde PM, Lipchik RJ, Rizzo JD, et al. Risk factors for bronchiolitis obliterans in allogeneic hematopoietic stem-cell transplantation for leukemia. Chest. 2005. 128:153–161.

7. Chien JW, Martin PJ, Gooley TA, Flowers ME, Heckbert SR, Nichols WG, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003. 168:208–214.

8. Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant. 2003. 9:657–666.

9. Majhail NS, Schiffer CA, Weisdorf DJ. Improvement of pulmonary function with imatinib mesylate in bronchiolitis obliterans following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006. 12:789–791.

10. Fullmer JJ, Fan LL, Dishop MK, Rodgers C, Krance R. Successful treatment of bronchiolitis obliterans in a bone marrow transplant patient with tumor necrosis factor-alpha blockade. Pediatrics. 2005. 116:767–770.

11. Smith EP, Sniecinski I, Dagis AC, Parker PM, Snyder DS, Stein AS, et al. Extracorporeal photochemotherapy for treatment of drug-resistant graft-vs.-host disease. Biol Blood Marrow Transplant. 1998. 4:27–37.

12. Alcindor T, Gorgun G, Miller KB, Roberts TF, Sprague K, Schenkein DP, et al. Immunomodulatory effects of extracorporeal photochemotherapy in patients with extensive chronic graft-versus-host disease. Blood. 2001. 98:1622–1625.

13. Rabitsch W, Deviatko E, Keil F, Herold C, Dekan G, Greinix HT, et al. Successful lung transplantation for bronchiolitis obliterans after allogeneic marrow transplantation. Transplantation. 2001. 71:1341–1343.

14. Gascoigne A, Corris P. Lung transplants in patients with prior bone marrow transplants. Chest. 1994. 105:327.

15. Svendsen UG, Aggestrup S, Heilmann C, Jacobsen N, Koch C, Larsen B, et al. Transplantation of a lobe of lung from mother to child following previous transplantation with maternal bone marrow. Eur Respir J. 1995. 8:334–337.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download