Abstract
Wernicke's encephalopathy is an acute neurolopsychiatric syndrome caused by thiamine deficiency, and classically presents with the triad of opthalmopathy, ataxia and altered mentality. Both prolonged total parenteral nutrition and reduced oral intake can induce Wernicke's encephalopathy during hematopoietic stem cell transplantation (HSCT). Although early treatment is important for recovery from Wernicke's encephalopathy, the vague symptoms and characteristics hinder early diagnosis. Furthermore, Wernicke's encephalopathy is not infrequent and can develop at any age during HSCT. Herein, we present two young patients developing Wernicke's encephalopathy during HSCT.
Wernicke's encephalopathy is an acute neurolopsychiatric syndrome that was first described in 1881 by Carl Wernicke. Its symptoms include the classical triad of opthalmopathy, ataxia and an altered mental status.1 It has generally been associated with alcoholism and thiamine deficiency, but it is now found in a variety of clinical settings.1 We experienced two cases of Wernicke's encephalopathy after prolonged period of total parenteral nutrition (TPN), after allogeneic bone marrow transplantation (BMT).
A 10-year-old boy was admitted to the hospital for an allogeneic BMT. He was diagnosed with non-Hodgkin's lymphoma 6 years previously, and the therapeutic procedure for the lymphoma was completed in October 2003. His disease relapsed in August 2007, and he was re-diagnosed with acute lymphoblastic leukemia based on bone marrow aspiration and biopsy. Complete remission was achieved, and an allogeneic BMT was planned. The patient underwent total body irradiation and cyclophosphamide for the conditioning; tacrolimus and methotrexate were used for the prophylaxis of graft versus host disease (GVHD) and he was provided with an immunosuppressant. His oral intake was low, and he was solely dependent on a commercially available total parenteral nutrition product. His neutrophils were engrafted on D+11. On D+11, he complained of dizziness and headache, but those symptoms quickly subsided. On D+23, vertigo and nystagmus developed, but his mental status was alert, and he did not show sensory or motor changes in the extremities. Three days later, he presented diplopia and esotropia, and complained of difficulty in closing his eyes. Brain magnetic resonance imaging (MRI) was performed; we initially considered the toxicity of metronidazole or immunosuppressant (Fig. 1A). Metronidazole administration was discontinued. On D+30, he started to complain of dyspnea, and suffered simple and complex partial seizures one day later, which composed of lip smacking and clonic movements of the left shoulder. Apnea and bradycardia developed on D+33 and the patient was transferred to the intensive care unit for ventilator care. His mental status became semicomatose. The follow-up brain CT and clinical progress since metronidazole discontinuation suggested Wernicke's encephalopathy (Fig. 1B). The blood level of thiamine was low (0.2 U/L; reference range 2-7 U/L). Thiamine was subsequently administered. Over the following two weeks, his mental status gradually, but not completely, improved. On D+50, he died of septic shock after suffering pneumonia, which developed after microaspiration.
A 12-year-old boy underwent allogeneic BMT. He was diagnosed with Philadelphia chromosome positive acute lymphoblastic leukemia. After induction and consolidation chemotherapy, the patient underwent the BMT. He received total body irradiation and cyclophosphamide administration for the conditioning procedure. Cyclosporine and methotrexate were used for the prevention of GVHD. He suffered from severe nausea and vomiting and could not orally take nutrition. On D+12, neutrophils were engrafted stably. After the conditioning procedure was initiated, the patient was solely dependent on a commercially available parenteral nutrition product. His general activity was slightly poor. He responded slowly to requests, but his mental status was alert. On D+22, he seemed slightly drowsy and confused, and complained of marked dizziness. He could not walk to toilet and intermittently exhibited intention tremors on both hands. Lid nystagmus was observed, but there was no eyeball nystagmus. There were no sensory or motor changes. MRI taken on D+24 revealed increased signal intensity on the dorsal pons on T1 weighted images (Fig. 2A). He was suspected of having Wernicke's encephalopathy, and thiamine 100 mg was given for 7 days. Twenty-four hours after thiamine infusion began, his dizziness and mental status were remarkably improved, and all his symptoms cleared four days later (Fig. 2B).
Thiamine, in its biologically active form thiamine pyrophosphate, is an essential coenzyme used in the tricarboxilic acid cycle and the pentose phosphate shunt for the metabolism of carbohydrates and lipids. This is an important enzyme in energy metabolism involving transketolase, alpha ketoglutarate dehydrogenase, and pyruvate dehydrogenase. Without thiamine, the overwhelming metabolic demand of brain cells leads to cellular energy deficit, focal acidosis, ultimate cell death,2 and lack of external balance. The requirement of thiamine is increased in critically ill patients or children and during lactation or pregnancy.1,3 Since the body's store of thiamine is depleted after roughly 20 days of poor supplementation, Wernicke's encephalopathy can develop between days +13 to +37 after BMT as in our cases and other reports.4,5
Wernicke's encephalopathy commonly develops in alcoholics as a result of thiamine deficiency, although it remains largely underdiagnosed in this group. Typical brain lesions are observed in 0.8-2% of unselected autopsies of alcoholics, but only 1-20% of these lesions are diagnosed clinically.6 Diagnosis of the disease is sometimes difficult because of non-specific initial symptoms such as headache, abdominal discomfort and fatigue; furthermore, the classic triad of symptoms are observed only in about 16% of patients.1 Without thiamine treatment, the disease incurs 17-20% mortality and progresses to Korsakoff's syndrome with memory impairment in 80% of cases.7 Progression to Korsakoff's syndrome is reduced among non-alcoholic Wernicke's encephalopathy patients compared to alcoholic ones. When Wernicke's encephalopathy is due to thiamine deficiency alone, Korsakoff's psychosis rarely develops following thiamine replacement. However, successful treatment or prophylaxis of Wernicke's encephalopathy depends on a number of related issues and is not simply a matter of supplementing thiamine.2 While thiamine replacement is important for the treatment and prophylaxis of Wernicke's encephalopathy, an effective dosing template has not yet been established.8
Recently, Wernicke's encephalopathy has been observed in a variety of cases not involving non-alcoholic patients, such as those undergoing gastrointestinal surgery, chronic diarrhea, hyperemesis gravidarum, anorexia nervosa, and cancer, etc.1 TPN without supplementation of thiamine sometimes causes Wernicke's encephalopathy. The risk of thiamine deficiency increases when physicians fail to check the composition of commercially available TPN products.1,5,9,10 Since a commercial form of TPN was first introduced to this hospital in 2004, there have been two severe cases of Wernicke's encephalopathy following BMT.
Prior to this report, two cases of Wernicke's encephalopathy during hematopoietic stem cell transplantation (HSCT) have been reported in Korea, and both were adults.10,11 Both cases underwent HSCT and prolonged TPN, and fully recovered from Wernicke's encephalopathy after thiamine infusion. Our patients were much younger than those in the previous reports. Considering the fact that many cases of Wernicke's encephalopathy are undiagnosed among alcoholics and there have globally been several reports of Wernicke's encephalopathy developing during HSCT, we suggest that Wernicke's encephalopathy can develop at any age and is not infrequent during HSCT.
Wernicke's encephalopathy is difficult to diagnose following BMT because its initial symptoms are not easily differentiated from other encephalopathies following BMT. Moreover, numerous therapeutic agents, including immunosuppressants and antibiotics, potentially affect the neurological systems.12 In an autopsy study, 5.5% (10/180) of cases were found to have Wernicke's encephalopathy.13 The characteristic findings of Wernicke's encephalopathy on MRI can be helpful for diagnosis; lesions are found often in the thalamus and mammilary body, but the tectal plate and cranial nerve nucleus may be involved in non-alcoholic Wernicke's encephalopathy.14 The involved lesions may share characteristics with metronidazole encephalopathy.15 Less common MRI imaging features of Wernicke's encephalopathy include lesions in the cerebellar vermis, whereas the cerebellar vermis is involved in a high percentage (85%, 17/20) of metronidazole encephalopathy cases. Metronidazole encephalopathy usually develops after a long period of use (11-52 days), and symptoms improve over a relatively short period (4-10 days) after the discontinuation of metronidazole.15 In our cases, discontinuation for 10 days was not effective in case 1; and metronidazole was used only for four days, long before the onset of symptoms in case 2. In both cases, cerebellar imaging suggested normal activity. Therefore, both clinical progressions of the two cases and their MRI imaging results suggested Wernicke's encephalopathy rather than metronidazole encephalopathy.
Chemotherapeutic agents might hamper the metabolism of thiamine and induce thiamine deficiency. Thiamine deficiency associated with chemotherapy is usually observed during or shortly after chemotherapy. Antimetabolites, especially 5-fluorouracil may cause thiamine deficiency, as it increases the rate of thiamine metabolism16 and because of the blockage of the Krebs cycle by fluoroacetate, a metabolite of 5-fluorouracil.17 On the other hand, thiamine pyrophosphate inhibits the cytotoxicity of methotrexate and cyclophosphamide.18
The importance of early diagnosis and treatment is clearly evident in the two cases presented herein. Ocular symptoms resolved within a few hours after treatment, and mental status improved over the following 2-3 weeks.1 In case 1, treatment for thiamine deficiency was provided 10 days after the onset of vertigo and headaches. Mental activity improved after thimanine replacement, but did not fully recover. Furthermore, the patient experienced breathing difficulties and ultimately cardiac arrest. In case 2, thiamine was provided immediately after its deficiency was suspected, and a full recovery was achieved.
In conclusion, Wernicke's encephalopathy may develop at any age and not infrequently after HSCT. Wernicke's encephalopathy should be considered in patients with encephalopathy following BMT or prolonged total parenteral nutrition without adequate oral intake. Early treatment improves the prognosis of Wernicke's encephalopathy cases. A multivitamin and thiamine should be provided for patients who depend solely on TPN to prevent Wernicke-Korsakoff syndrome.
Figures and Tables
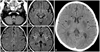 | Fig. 1(A) Symmetric signal increase on fast fluid-attenuated inversion-recovery images in the dorsal part of the medulla oblongata (black arrow, A1) same signal changes at the right side colliculus of the midbrain (arrow head, A2), the right precentral cortex (thin arrow, A3) and the bilateral putamen (white arrow, A4). (B) Non-contrast computed tomography scan taken 7 days later, shows low density lesions in the bilateral putamen (black arrow) and newly developed lesions in the bilateral caudate nucleus (arrow head). |
 | Fig. 2(A) Increased signal intensity on the bilateral medial lemnisci of the dorsal pons (arrow, T2 weighted image, A1) and the colliculi of the dorsal midbrain (arrow head, FLAIR, A2). (B) Improved lesions following 7 days of intravenous thiamine supplement (T2 weighted image and FLAIR, B1 and B2). FLAIR, fluid-attenuated inversion-recovery. |
References
1. Sechi G, Serra A. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007. 6:442–455.

2. Thomson AD, Marshall EJ. The natural history and pathophysiology of Wernicke's Encephalopathy and Korsakoff's Psychosis. Alcohol Alcohol. 2006. 41:151–158.

4. Francini-Pesenti F, Brocadello F, Manara R, Santelli L, Laroni A, Caregaro L. Wernicke's syndrome during parenteral feeding: not an unusual complication. Nutrition. 2009. 25:142–146.

5. Bleggi-Torres LF, de Medeiros BC, Ogasawara VS, Loddo G, Zanis Neto J, Pasquini R, et al. Iatrogenic Wernicke's encephalopathy in allogeneic bone marrow transplantation: a study of eight cases. Bone Marrow Transplant. 1997. 20:391–395.

6. Torvik A. Wernicke's encephalopathy--prevalence and clinical spectrum. Alcohol Alcohol Suppl. 1991. 1:381–384.
8. Day E, Bentham P, Callaghan R, Kuruvilla T, George S. Thiamine for Wernicke-Korsakoff Syndrome in people at risk from alcohol abuse. Cochrane Database Syst Rev. 2004. CD004033.

9. Hahn JS, Berquist W, Alcorn DM, Chamberlain L, Bass D. Wernicke encephalopathy and beriberi during total parenteral nutrition attributable to multivitamin infusion shortage. Pediatrics. 1998. 101:E10.

10. Baek JH, Sohn SK, Kim DH, Kim JG, Lee HW, Park SP, et al. Wernicke's encephalopathy after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005. 35:829–830.

11. Choi YJ, Park SJ, Kim JS, Kang EJ, Choi CW, Kim BS. Wernicke's encephalopathy following allogeneic hematopoietic stem cell transplantation. Korean J Hematol. 2010. 45:279–281.

12. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008. 29:1036–1042.

13. Bleggi-Torres LF, de Medeiros BC, Werner B, Neto JZ, Loddo G, Pasquini R, et al. Neuropathological findings after bone marrow transplantation: an autopsy study of 180 cases. Bone Marrow Transplant. 2000. 25:301–307.

14. Zuccoli G, Santa Cruz D, Bertolini M, Rovira A, Gallucci M, Carollo C, et al. MR imaging findings in 56 patients with Wernicke encephalopathy: nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol. 2009. 30:171–176.

15. Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol. 2007. 28:1652–1658.

16. Kondo K, Fujiwara M, Murase M, Kodera Y, Akiyama S, Ito K, et al. Severe acute metabolic acidosis and Wernicke's encephalopathy following chemotherapy with 5-fluorouracil and cisplatin: case report and review of the literature. Jpn J Clin Oncol. 1996. 26:234–236.

17. Koenig H, Patel A. Biochemical basis for fluorouracil neurotoxicity. The role of Krebs cycle inhibition by fluoroacetate. Arch Neurol. 1970. 23:155–160.
18. Boros LG, Brandes JL, Lee WN, Cascante M, Puigjaner J, Revesz E, et al. Thiamine supplementation to cancer patients: a double edged sword. Anticancer Res. 1998. 18:595–602.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download