Abstract
Purpose
Although the rectus abdominis and its sheath are well known structures, their development in the human fetus is poorly understood.
Materials and Methods
We examined rectus abdominis and sheath development in semiserial horizontal sections of 18 fetuses at 5-9 weeks of gestation.
Results
Rectus muscle differentiation was found to commence above the umbilicus at 6 weeks and extend inferiorly. Until closure of the anterior chest wall via fusion of the bilateral sternal anlagen (at 7 weeks), the anterior rectal sheath originated from the external oblique and developed towards the medial margin of the rectus abdominis at all levels, including the supracostal part. After formation of the anterior sheath, fascial laminae from the internal oblique and transversus abdominis contributed to formation of the posterior rectus sheath. However, the posterior sheath was absent along the supracostal part of the rectus abdominis, as the transversus muscle fibers reached the sternum or the midline area. Therefore, it appeared that resolution of the physiological umbilical hernia (8-9 weeks) as well as chest wall closure was not required for development of the rectus abdominis and its sheath. Conversely, in the inferior part of the two largest fetal specimens, after resolution of the hernia, the posterior sheath underwent secondary disappearance, possibly due to changes in mechanical stress.
The rectus abdominis extends between the rib cage and pubis, and is supplied by the lower intercostal and corresponding segmental abdominal nerves (Th7-L1). According to Gray's Anatomy,1 at the superior end, the rectus abdominis is attached by three slips of muscle to the fifth, sixth and seventh costal cartilages. Thus, the rectus abdominis carries a distinct supracostal part. However, a limited study using mice and rats2 indicated development commenced earlier than both chest wall closure and resolution of the physiological umbilical hernia. The muscle fibers of the rectus abdominis are interrupted by three fibrous bands or tendinous intersections at specific sites above and at the level of the umbilicus.1 Gościcka and Murawski3 reported that the rectus muscle fibers appeared at 10 weeks of gestation, the epigastric arteries at 13-14 weeks, and the tendinous intersections at around 18 weeks. Despite the above-mentioned studies, fetal development of the rectus abdominis remains poorly understood.
The rectus sheath of the anterior abdominal wall is composed of three aponeurotic laminae or leaves with decussating fibers originating from the external oblique (EO), internal oblique (IO) and transverses abdominis (TA): the lamina from the EO joins the anterior rectus sheath, that from the TA connects with the posterior rectus sheath, and the laminae from the IO merge with both sheaths above the umbilicus.1 The posterior sheath is absent below the umbilicus because all laminae join mutually to form the anterior sheath. Although Niikura, et al.4 demonstrated that the posterior sheath was absent below the umbilicus in human fetuses at 20-25 weeks, this was not the focus of their investigation. For the sheath above the umbilicus, Rizk5,6 postulated the so-called "bilaminar theory": a classical lamina from each of the EO and IO is divided into two laminae according to the collagen fiber direction. That theory provided a better biomechanical model for the adult rectus sheath as an intermediate tendon of the bilateral abdominal wall muscles. However, our interpretation of the rat fetus findings of Rizk and Adieb7 is that they failed to demonstrate fetal development of the rectus sheath. Differentiation in the bilaminar and decussating collagen fibers in the rectus sheath5,6 may be established by mechanical demands after birth.
Instead of the sternalis, a well-known variant of the supracostal rectus muscle, sometimes innervated by the intercostal nerve,8-12 we speculated that in fetuses the rectus abdominis might extend more superiorly along the rib cage compared to adults. Consequently, the aim of the present study was to improve understanding of the fetal development of the rectus abdominis muscle and its associated sheath in humans. We followed the development of those structures in stained sections derived from human embryos and fetuses aged between 5 and 9 weeks of gestation. Of interest to surgeons, we also addressed fetal development of the inferior epigastric artery, especially that of the medial and lateral rows of the musculocutaneous perforators.13
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We examined paraffin-embedded histological sections derived from 18 embryos and fetuses at 5-9 weeks of estimated gestational age: three embryos at each of 5 weeks [Crown-Rump Length (CRL) 7-10 mm] and 6 weeks (CRL 15-17 mm), and four embryos or fetuses at each of 7 weeks (21-25 mm), 8 weeks (27-29 mm) and 9 weeks (31-36 mm). All sections were horizontal, 5 microns thick, prepared at intervals of 20 microns, and stained with hematoxylin and eosin or silver impregnation. This work was a qualitative study with no specific measures and we did not use any specific instrument other than a light microscope.
All specimens were part of the collection of the Embryology Institute of Universidad Complutense, Madrid. Specimens were from women who had had miscarriages or ectopic pregnancies at the Department of Obstetrics of the University. We were unable to rule out the presence of pathologies due to the nature of the specimens. However, the study sought to identify the morphology most commonly evident in fetuses at each stage. Approval for the study was granted by the ethics committee of the University (No. B08/374).
In all figures, the upper side of each panel corresponds to the anterior or ventral side of the body. Apart from the two largest specimens (CRL 36 mm), all specimens were at stages of development prior to resolution of the physiological umbilical hernia. Moreover, anterior chest wall closure was completed through fusion of the lower ends of the bilateral sternal anlagen (the so-called sternal bands14) at 8-9 weeks. Thus, other than 4 specimens at 9 weeks, the midline area of the lower chest wall, in front of the liver or heart, was filled with loose mesenchymal tissue covered by the surface ectoderm. We found that the rectus abdominis and its sheath commenced development at early stages in which both the herniated gut and the loose tissue covering of the liver and heart were seen.
In three specimens at 5 weeks, there were two plate-like mesenchymal condensations in the abdominal wall. Those two condensations appeared to correspond to the undifferentiated rectus abdominis and lateral abdominal muscles, respectively (Fig. 1). We regarded the midline condensation as the so-called "abdominal band" reported by Munger and Munger2 in fetal mice and rats. The candidate rectus muscle anlage had already extended longitudinally from the level of the inferior margin of the brachial plexus to the level below, but immediately above the umbilicus. At the earliest stage, neither the muscle fibers nor the rectus sheath was seen. The developing ribs were still restricted in the posterolateral part of the body wall. In two of three specimens at 6 weeks (Fig. 2), the rectus abdominis, EO and IO muscles were identified as independent muscle sheets at the level above the umbilicus (strictly, above the umbilical vein exit from the liver). However, the transversus abdominis and thoracis were difficult to identify. The rectus sheath was not present. A thick, loose mesenchymal tissue layer existed between the EO and the thin surface ectoderm. The diaphragm was not yet present at the posterior side of the adrenal, while the pleural cavity extended inferiorly as a loose mesenchymal space between the peritoneum and the posterior body wall.
The abdominal muscles and their associated connective tissues appeared to be differentiated between 6 and 7 weeks. At that time, the bilateral anlagen of the sternum had begun to unite with the superior part (i.e., the manubrium), but it had not reached the inferior part. However, in three of four specimens at 7 weeks, the rectus abdominis had already extended between the laterally located sternum and the pubis without any interruption alongside the umbilical hernia. Moreover, at 7 weeks, the supracostal part of the rectus abdominis was consistently seen at the superficial side of the internal intercostal (Fig. 3). In all four specimens at 7 weeks, above the level of the umbilical vein exit from the liver, the connective tissue laminae extended medially from each of the EO, IO and transverse muscles. Thus, development of the sheath started from the superior part. The development of the anterior rectus sheath was followed by that of the posterior sheath. At the early stage, the anterior sheath did not reach the medial margin of the rectus abdominis, while the posterior sheath was difficult to discriminate from a mesenchymal condensation along the primitive peritoneum (Fig. 3B-E). The connective tissue lamina from the EO consistently covered the supracostal rectus muscle. The skin differentiated from the surface ectoderm. At the superior margin, the rectus abdominis was adjacent to the pectoralis major, which was developing from the superolaterally located shoulder. The transverses abdominis was continuous with the transverses thoracis and, at the ribs and sternum, the diaphragm insertions were interdigitated with the two transversus muscles. The inferior epigastric artery was identified at 7 weeks (Fig. 3G). However, ramification of this artery was not seen at this stage. In addition, the rectus abdominis was very narrow along the mediolateral axis at the level below the umbilicus, and the attachment to the developing pubis appeared to be single (not two in number; Fig. 3G insert).
At 8-9 weeks, fusion of the bilateral anlagen of the sternum was completed, and the umbilical hernia had resolved. At that time, the rectus abdominis increased in thickness and the anterior and posterior sheaths reached the medial margin of the rectus abdominis (Fig. 4) and completely enclosed it (Figs. 5 and 6). Simultaneously, the superior and inferior epigastric arteries became clear along the posterior aspect of the rectus abdominis. In four fetuses at 9 weeks, the inferior epigastric artery divided into two major braches below the umbilicus in a single specimen (Fig. 5F) and above the umbilicus in three specimens (Fig. 6D-F). In regards to the muscle, the medial end facing the herniated gut was especially thick (Fig. 5E). The posterior sheath was clearly seen originating from the transverses abdominis and IO (Figs. 4E and 5F). However, the posterior sheath was absent along the supracostal part of the rectus abdominis, as the muscle belly of the transverses abdominis/thoracis reached the midline area to insert into the sternum with or without a short aponeurosis. Although the internal intercostal occupied the intercostal space, we were unable to discriminate the innermost intercostal from the internal intercostal in the present sections. The 11th rib received the internal oblique at levels of the umbilical hernia (Fig. 5E). Sometimes (3 of 8 specimens at 8-9 weeks), the rib cage did not reach an inferior level, including the umbilical vein exit from the liver. The linea alba was composed of transversely directing fibrous tissues at 8-9 weeks (Figs. 4F and 6G, H).
Notably, in the inferior part of the two largest specimens (CRL 36 mm) (Fig. 6G), the posterior sheath was in the process of disappearing along the course of the inferior epigastric artery and vein. At this site, the umbilical artery did not directly attach to the anterior body wall, but there was a mesentery-like structure connecting with a limited, midline area (Figs. 4F and 6F). We did not find any tendinous intersections of the rectus abdominis; however, the horizontal sections used in the study were not ideal for making such an observation. Likewise, we did not find two heads of the muscle near or at the attachment to the pubis (Fig. 6H). At 8 weeks (Fig. 4), abundant vessels appeared in the developing subcutaneous tissue between the EO and skin. Overall, rectus abdominis differentiation was found to commence above the umbilicus at 6 weeks, and extended inferiorly. Thus, the muscle was absent or thin in the levels below the umbilicus until 8 weeks: the inferior part increased its width and thickness at 9 weeks. Conversely, it did not develop from a two-headed origin1 at or near the pubis.
The present study indentified a candidate primitive rectus abdominis as a plate-like mesenchymal condensation at the anterior midline area in the smallest embryos studied (CRL 7 mm) (Fig. 1). We believed the midline condensation to be analogous to the so-called "abdominal band" previously reported by Munger and Munger2 in their study of fetal mice and rats. However, the anlage remained restricted above the umbilicus, reaching the level near the brachial plexus. Moreover, in the next stage, the rectus abdominis was not seen near the midline but was observed in the relatively lateral part of the body wall. Accordingly, we wondered how it is possible that the midline condensation or abdominal band develops into the "laterally" located rectus muscle. The abdominal band shown in Fig. 1 might be a mesenchymal condensation not for the rectus abdominis but for the ventral epidermis. If so, that band may have a different role in humans compared to mice and rats. Rozen, et al.13 introduced a theory of rectus abdominis muscle development: 1) the ventral longitudinal column containing myogenic cells or the primitive rectus abdominis divides into the "two heads of origin" comprising an attachment at the pubic symphysis medially and that from the upper border of the pubic crest laterally; 2) the two muscle slips develop cranially to reach the costal cartilage. However, according to the present results, rather than the inferior part near the pubic bone anlage, the muscle is most likely to start development above the umbilicus, especially in front of the liver at 7 weeks. Thus, the developing rectus abdominis seemed to require mechanical support from the posterior side, by the liver and ribs. The rectus muscle below the umbilicus increased its width and thickness at 9 weeks. Nevertheless, we have no evidence of the two-heads near the pubic bone among the specimens examined. According to Niikura, et al.,4 the most inferior part of the rectus abdominis is not composed of two heads at even 25 weeks. Thus, the two definite heads or origins, if present in adults, may develop after birth.
Apart from two specimens of CRL 36 mm, all of the present specimens were at stages of development prior to closure of the anterior abdominal wall or before resolution of physiological umbilical hernia. Conversely, the rectus abdominis and its sheath commenced developing in specimens with a herniated gut. It may be that below the umbilicus, even after resolution of hernia, the urachus and umbilical arteries exert a negative mechanical effect on the development of the posterior rectus sheath. However, without closure of the abdominal and chest walls, the anterior (or posterior) sheath could develop as an insertion of the external oblique (or the transversus abdominis). Otherwise, closure of the anterior chest wall and resolution of the hernia are likely to cause a topographical change in the rectus abdominis from the early lateral site to the final midline area. Growth of the ribs and of the lower limb may also contribute to the anteromedial migration of the rectus abdominis.
We found that after the physiological umbilical hernia had resolved, the posterior sheath seemed to secondarily disappear below the umbilicus along the inferior epigastric artery. The umbilical artery was separated from the missing sheath by the peritoneal cavity. We first considered that developing fatty tissue was committed to the secondary disappearance of the posterior sheath, but the fatty tissue appeared at a much later stage, such as at 15 weeks.4 Changes in mechanical stress after hernia resolution seemed to induce this disappearance. These observations together with those of Niikura, et al.4 suggest that the posterior sheath below the umbilicus completely disappears by 25 weeks of gestation. At the stage just after the herniated gut returned, the linea alba began to develop as a transversely directed fibrous tissue. Thus, resolution of the hernia could be an important accelerating factor in the differentiation of connective tissues in the anterior midline area, albeit only one of a few factors. Rizk's bilaminar sheaths, as well as his proposed digastric configuration of the abdominal muscles,5,6 may result from functional adaptation to mechanical demands during trunk muscle function after birth.
The large umbilicus appears to stop myotomal cell migration in order to make a pair of myogenic cell pools or the abdominal band.2 The band seems to be mechanically strong enough to receive the anterior sheath from the external oblique, although the liver also supports the inner aspect. We interpreted the figures of Manley, et al.15 to indicate that even in specimens with unsuccessful fusion of the bilateral anlagen of the sternum in hoxb4 mutant mice, the rectus muscle or the sternalis (a variant of the pectoralis: see below) seems to develop. However, in AP-2alpha transcription factor knockout mice, the abdominal band as well as the covering abdominal epidermis failed to develop.16 If gastroschisis (i.e., unsuccessful fusion of the bilateral upper abdominal walls) is due to such a loss of transcription factor function, the rectus abdominis may also not develop. However, if gastroschisis is the result of anomalous yolk sac development,17,18 we may be able to identify the rectus abdominis due to early development before hernia resolution. According to Munger and Munger,2 all developmental events involving nerves, vessels and muscles in the anterior abdominal wall are linked to a process of differentiation of the epidermis. However, in the present study, the rectus abdominis and its sheath commenced development prior to skin differentiation.
Muscle fibers in the anterior abdominal wall muscles originate from somites,19 whereas the associated connective tissues originate from the lateral plate because myogenic cells migrate laterally beyond the lateral somitic frontier.20 Such muscles have recently been termed abaxial muscles, rather than the classical name hypaxial muscles.21,22 Therefore, the rectus sheath also seems to originate from the lateral plate. However, the rectus sheath is unlikely responsible for the pattern of formation of the muscle, but is rather more likely to form later under mechanical influence of the three-layered lateral muscles. Notably, at the earliest stage, the superior part of the rectus abdominis was at the same level as the brachial plexus. The rectus abdominis suddenly stopped upward extension at the margin of the inferiorly developing pectoralis major. Thus, the layer or position of the rectus abdominis seemed to correspond to that of the pectoralis at the superficial side of the anterior chest wall. The external intercostal was outside of the competitive relationship for the common territory between the pectoralis and rectus abdominis. According to Evans, et al.,23 in contrast to non-migratory lateral abdominal muscles, the rectus muscle anlage migrates along the body wall, similar to the pectoralis muscle anlage.
Using a chick-quail chimera system, Liem and Aoyama22 recently demonstrated that an ectopic limb experimentally induced on the superficial side of the lower chest wall often interfered with development of the rectus abdominis. The pectoralis-like muscle was observed to additionally appear in the original abdominal wall instead of the rectus abdominis, even though the chick rectus abdominis is derived from only 1 or 2 segmental anlagen. The rectus thoracis, if present in humans (e.g., Mehta, et al.24), is likely to correspond to part of the pectoralis major such as the sternalis. The sternalis, a well-known variant similar to the supracostal part of the rectus abdominis, is supplied by the pectoral nerve(s).25,26 O'Neill and Folan-Curran27 reported on the sternalis with muscle fibers that directly communicated with the pectoralis, and various patterns were reviewed by Jelev, et al.28 The sternalis was also reported in combination with the absence of the sternocostal part of the pectoralis.29-31 Moreover, similar to the rectus abdominis, the sternalis may sometimes be innervated by the intercostal nerve.8-12 Since the rectus-like or sternalis-like muscle appears in hoxb4 mutant mice along with failure of the bilateral sternal anlagen (Manley, et al.15; see above), the common variant is likely to result from a slight discrepancy in timing between chest wall closure and the inferomedially developing pectoralis major. We concluded that in fetal development as well as in evolution, the pectoralis interferes with supracostal expansion of the rectus abdominis.
Finally we discussed the medial and lateral perforator rows of the inferior epigastric artery that is of great interest to surgeons.13 Rozen, et al.13 considered that, because there are two muscle heads very early on in its development, each head draws its own blood supply to provide "a perforator row" for each head. However, we have provided evidence against this possibility (see the first paragraph of the Discussion). In short, if we use the terms of "origin and insertion" for anatomical description of muscles, the rectus abdominis seemed to develop from its insertion to its origin, not from the origin to the insertion. In three of four of the present specimens at 9 weeks, in which the rectus abdominis grew rapidly at the level below the umbilicus, the inferior epigastric artery divided into two major branches at the level above the umbilicus. Although the present specimens were very limited in number, this variation appears not to be so different from that known in adults.13 The two-row arrangement of the perforators seems to depend on a fact that there are two major arterial branches running longitudinally in fetuses. The number of branches may depend on fetal muscle thickness and width, not on the hypothetical two columnar anlagen of the rectus abdominis.
Figures and Tables
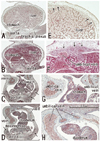 | Fig. 1The abdominal wall is occupied by the umbilical veins in tilted horizontal sections of a 5-week embryo (CRL 7 mm). HE staining. Panel A (or Panel D) is the most superior (or inferior) in the figure. Panels E-H are higher magnification views of the primitive rectus abdominis shown in panels A-D, respectively. Panel C displays the level of the umbilical hernia. Arrows in panels B-D (or E and F) indicate developing ribs (or candidate anlagen of the abdominal wall muscles). The plate-like, midline condensation (arrows in panel F) appears to correspond to the abdominal band in mice and rats (see text for explanation). Panels A-D or panels E-H were prepared at the same magnification (scale bar in panels D and H). |
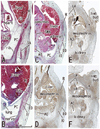 | Fig. 2Abdominal wall muscles appear in horizontal sections of a 6-week embryo (CRL 16 mm). HE staining (panels A-C) and silver impregnation (panels D-F). Panel A (or Panel F) is the most superior (or inferior) level of the figure. The rectus abdominis (R) appears to be differentiated at levels above the umbilicus (panels A and B), but it is identified as a mesenchymal cell cluster alongside the umbilicus (panels C and D) and is difficult to identify below the umbilicus (star in panels E and F). Likewise, the oblique muscles (EO, IO) are not identified below the umbilicus (panels E and F). The rectus abdominis is located in the lateral part of the body wall rather than the anterior site. The rectus sheath (arrowheads) was not seen at any level. The diaphragm has not yet developed. The future inferior part of the pleural cavity is filled with loose tissue (PC in panels A-C). All panels were prepared at the same magnification (scale bar in panel B). In all figures including Fig. 1, the upper side of each panel corresponds to the anterior or ventral side of the body. AO, aorta; SA, serratus anterior; SMA, superior mesenteric artery; UA, umbilical artery; UR, urachus; EO, external oblique; IO, internal oblique; PC, primitive pleural cavity. |
 | Fig. 3Anterior rectal sheath appears in horizontal sections of a 7-week embryo (CRL 21 mm). HE staining. Panel A (or Panel G) is the most superior (or inferior) level of the figure. The rectus abdominis extends superiorly in the superficial side of the rib cage (panels A-D). However, the anterior chest wall is not closed by the sternum but by the surface ectoderm and underlining mesenchymal tissues (stars in panel B). Panels C and D are higher magnification views of the bilateral rectus muscles shown in panel B. The PC is filled with loose mesenchymal tissue (panels B and C). Panel E, the level of the umbilical vein exiting the liver, with the ribs also present. The anterior sheath (arrowheads), originating from the external oblique (EO), covers the rectus abdominis at levels above the umbilicus (panels B-E). In panel F (the level of the umbilical hernia) and panel G (a level below the umbilicus), the rectus sheath as well as the transversus (T) is difficult to identify. In panel G, the rectus abdominis is artifactually divided into two parts during the histological procedure. The rectus abdominis is reduced in size at the level below the umbilicus (panel G). An insert of panel G demonstrates the most inferior part of the muscle comprising of a single small band adjacent to the pubic bone anlage (pubis). Panels A and C-G were prepared at the same magnification (scale bar in panel A). D, diaphragm; IEA, inferior epigastric artery; IO, internal oblique; PC, primitive pleural cavity; T, transverses abdominis/thoracis; UA, umbilical artery; UR, urachus. |
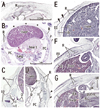
 | Fig. 4Posterior rectus sheath appears in horizontal sections of an 8-week embryo (CRL 29 mm). HE staining. Panel A (or Panel F) is the most superior (or inferior) level of the figure. In panels A-C, the rectus abdominis (R) attaches to the anterior aspect of the ribs, but it does not extend medially to reach the sternum (S). The rectus abdominis is located at the superficial side of the internal intercostal (II). The asterisk in panel A indicates bleeding. The anterior sheath (arrowheads), originating from the external oblique (EO), covers the rectus abdominis at all levels (panels A-F). In panel D, the level of the umbilical vein exiting the liver, note the absence of ribs. In panel E (a level of the umbilical hernia) and panel F (a level below the umbilicus), the posterior sheath (arrows) originates from the transversus abdominis (T). In panel F, the midline area is composed of loose mesenchymal tissue (asterisk) and the anterior sheath (arrowheads) does not reach the midline area. All panels were prepared at the same magnification (scale bar in panel A). D, diaphragm; IEA, inferior epigastric artery; IO, internal oblique; T, transverses abdominis/thoracis; UA, umbilical artery; UR, urachus. |
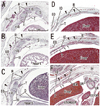
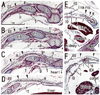 | Fig. 5Posterior rectus sheath becomes clear in horizontal sections of a 9-week fetus (CRL 35 mm). HE staining. Panel A (or Panel F) is the most superior (or inferior) level of the figure. The bilateral sternal anlagen have already fused (panels A and B). The supracostal part of the rectus abdominis (R) is located on the superficial side of (panel A), but in panels B and C, at the same layer as the internal intercostal (II). The anterior sheath (arrowheads), originating from the external oblique (EO), covers the rectus abdominis at all levels (panels A-F). In panel D, the level of the umbilical vein exiting the liver, the ribs are seen. A midline raphe-like structure can be seen (asterisk in panel D). Panel E, a level of the umbilical hernia, which includes the 11th rib that receives the internal oblique (IO). In panel F, a level below the umbilicus, three lateral muscles are separated by relatively wide loose mesenchymal tissues. Arrows in panels E and F indicate the posterior sheath originating from the transversus abdominis (T). All panels were prepared at the same magnification (scale bar in panel A). SMA, superior mesenteric artery. D, diaphragm; IEA, inferior epigastric artery; S, sternum. |
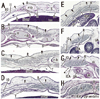 | Fig. 6Secondary change of the posterior rectus sheath after resolution of the umbilical hernia: horizontal sections of a 9-week fetus (CRL 36 mm). HE staining. Panel A (or Panel H) is the most superior (or inferior) level of the figure. In panels A-C, the rectus abdominis (R) is well developed in the anterior aspect of the internal intercostal (II). In panel D, the level of the umbilical vein exit, note the absence of ribs. The anterior sheath (arrowheads) is clearly identified at the medial side of the rectus abdominis (panels E-H). At a level below the umbilicus, part of the posterior sheath is absent (arrow with star in panel G) and the midline area (asterisk in panels G and H) is composed of dense connective tissue. The inferior epigastric artery (IEA) runs upward from panel H to panel F as a single trunk, and divides into two major branches at a level between panels E and F. All panels were prepared at the same magnification (scale bar in panel A). D, diaphragm; IO, internal oblique; T, transverses abdominis/thoracis; UA, umbilical artery; UR, urachus; EO, external oblique; S, sternum. |
ACKNOWLEDGEMENTS
This study was supported by a grant (0620220-1) from the National R & D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea.
References
1. Williams PL. Gray's Anatomy. 1995. 38th ed. Edinburgh: Churchill Livingstone;825–829.
2. Munger GT, Munger BL. Differentiation of the anterior body wall and truncal epidermis and associated co-migration of cutaneous nerves and mesenchyme. Anat Rec. 1991. 231:261–274.

3. Gościcka D, Murawski E. Tendinous intersections of the rectus abdominis muscle in human fetuses. Folia Morphol (Warsz). 1980. 39:427–434.
4. Niikura H, Okamoto S, Nagase S, Takano T, Murakami G, Tatsumi H, et al. Fetal development of the human gubernaculum with special reference to the fasciae and muscles around it. Clin Anat. 2008. 21:547–557.

5. Rizk NN. A new description of the anterior abdominal wall. Anat Rec. 1976. 184:515.
6. Rizk NN. A new description of the anterior abdominal wall in man and mammals. J Anat. 1980. 131(Pt 3):373–385.
7. Rizk NN, Adieb N. The development of the anterior abdominal wall in the rat in the light of a new anatomical description. J Anat. 1982. 134(Pt 2):237–242.
8. Rao VS, Rao GRKH. The sternalis muscle. J Anat Soc India. 1954. 3:49–51.
9. Kacker GN. Sternalis muscle in U.P. Indian subjects. J Anat Soc India. 1960. 9:101–103.
10. Blees G. A peculiar type of sternalis muscle. Acta Morphol Neerl Scand. 1968. 7:69–72.
11. Jeng H, Su SJ. The sternalis muscle: an uncommon anatomical variant among Taiwanese. J Anat. 1998. 193(Pt 2):287–288.

12. Saeed M, Murshid KR, Rufai AA, Elsayed SE, Sadiq MS. Sternalis. An anatomic variant of chest wall musculature. Saudi Med J. 2002. 23:1214–1221.
13. Rozen WM, Kapila S, Donahoe S. Why there are two rows of deep inferior epigastric artery perforators despite variability in the number of deep inferior epigastric artery trunks: an anatomical and embryological argument. Clin Anat. 2011. 24:786–788.

14. Klíma M. Early development of the human sternum and the problem of homologization of the so-called suprasternal structures. Acta Anat (Basel). 1968. 69:473–484.

15. Manley NR, Barrow JR, Zhang T, Capecchi MR. Hoxb2 and hoxb4 act together to specify ventral body wall formation. Dev Biol. 2001. 237:130–144.

16. Brewer S, Williams T. Loss of AP-2alpha impacts multiple aspects of ventral body wall development and closure. Dev Biol. 2004. 267:399–417.

17. Vermeij-Keers C, Hartwig NG, van der Werff JF. Embryonic development of the ventral body wall and its congenital malformations. Semin Pediatr Surg. 1996. 5:82–89.
18. Stevenson RE, Rogers RC, Chandler JC, Gauderer MW, Hunter AG. Escape of the yolk sac: a hypothesis to explain the embryogenesis of gastroschisis. Clin Genet. 2009. 75:326–333.

19. Christ B, Jacob M, Jacob HJ. On the origin and development of the ventrolateral abdominal muscles in the avian embryo. An experimental and ultrastructural study. Anat Embryol (Berl). 1983. 166:87–101.

20. Durland JL, Sferlazzo M, Logan M, Burke AC. Visualizing the lateral somitic frontier in the Prx1Cre transgenic mouse. J Anat. 2008. 212:590–602.

21. Nowicki JL, Takimoto R, Burke AC. The lateral somitic frontier: dorso-ventral aspects of anterio-posterior regionalization in avian embryos. Mech Dev. 2003. 120:227–240.

22. Liem IK, Aoyama H. Body wall morphogenesis: limb-genesis interferes with body wall-genesis via its influence on the abaxial somite derivatives. Mech Dev. 2009. 126:198–211.

23. Evans DJ, Valasek P, Schmidt C, Patel K. Skeletal muscle translocation in vertebrates. Anat Embryol (Berl). 2006. 211:Suppl 1. 43–50.

24. Mehta V, Arora J, Yadav Y, Suri RK, Rath G. Rectus thoracis bifurcalis: a new variant in the anterior chest wall musculature. Rom J Morphol Embryol. 2010. 51:799–801.
25. Ura R. Über die allgemeine Differenzierung der oberflächlichen Brustmuskeln mit besonderer Berűcksichtigung der Hautrumpfmuskeln der Säugetiere. Mitteil Med Gesellsch Tokyo. 1937. 51:216–288. 339–390.
27. O'Neill MN, Folan-Curran J. Case report: bilateral sternalis muscles with a bilateral pectoralis major anomaly. J Anat. 1998. 193(Pt 2):289–292.
28. Jelev L, Georgiev G, Surchev L. The sternalis muscle in the Bulgarian population: classification of sternales. J Anat. 2001. 199(Pt 3):359–363.

29. Kitamura S, Yoshioka T, Kaneda M, Matsuoka K, Chen KL, Sakai A. [A case of the congenital partial defect of the pectoralis major--accompanied by the sternalis with enormous size]. Kaibogaku Zasshi. 1985. 60:728–732.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download