Abstract
Purpose
The objective of this study is to evaluate the effects of heat and massage application on autonomic nervous system.
Materials and Methods
One hundred thirty-nine subjects volunteered and completed this study. Heat and massage was daily applied for 40 minutes, 5 days a week for 2 weeks. Primary-dependent measures included heart rate variability, sympathetic skin response, and serum cortisol and norepinephrine levels.
Results
Serum cortisol levels were significantly decreased at 2 weeks compared to baseline (p=0.003). Plasma norepinephrine levels at 4 weeks were significantly decreased compared to baseline (p=0.010). Heart rate, using the power spectra, increased significantly after 2 weeks compared to baseline. Of autonomic nerve conduction measures, latency was significantly increased at 2 and 4 weeks compared to baseline (p=0.023, 0.012), and amplitude was significantly decreased at 4 weeks compared to baseline (p=0.008). There were no serious adverse events such as burns or other major complications.
Stress causes mental, emotional, and physical responses in humans. When stressed, an organism first experiences arousal of the sympathetic nervous system, followed by activation of the musculoskeletal, cardiovascular, and endocrine systems. The outcome of this process is a series of non-specific psycho-physiologic changes, such as an increase of certain neurotransmitters in the bloodstream, and results in adverse effects associated with the negative aspect of stress. Psychologically, highly differentiated synthetic behavior often occurs as a result of the activation of cognitive and emotional processes. Accordingly, when an individual is stressed, they may feel emotions such as fear and anxiety together with physiological responses. If this stimulus is repeated, anxiety may become learned or conditioned, and the response may become generalized. At present, the precise mechanisms of the interaction between stress and the responses of various systems have not been fully elucidated, nevertheless, it is generally recognized that systemic responses may influence the maladaptive behavior of an organism in response to prolonged stress. For this reason, examining therapies that modulate stress responses in organ systems such as the musculoskeletal, nervous, and endocrine systems may be helpful in psychiatric illnesses such as depression and generalized anxiety disorder.1
Heat application is thought to enhance metabolism and facilitate circulation by dilating blood vessels and improving the functions of enzymes. These actions lead to increased catabolism, excretion of lactic acid, free fatty acids, and subcutaneous fat, and the removal of uric acid and other acidic waste products from muscle cells. By these mechanisms, heat therapy is thought to reduce fatigue and signs of aging, and to produce an analgesic effect. Heat is clinically effective for the management of various injuries and to relieve back, shoulder, and other types of musculoskeletal pain.2
To decrease allodynia and stress in clinical practice, massage is sometimes used as alternative management. When healthy volunteer or patients have been given massage, change in autonomic nervous system such as heart rate variability (HRV), blood pressure and respiratory rate have been observed. These results suggest that massage influences the autonomic nervous system and changes stress reactions. Despite recent researches in this field, however, only a few models of the effect of massage on stress responses have been reported.3 One hypothesis is that moderate massage can increase response in the autonomic nervous system by activating sensory receptors,4 and another is that touch massage influences to the release of oxytocin.5
The aim of this study was to evaluate the effects of heat and massage application on autonomic response in relation to stress, as measured by HRV, sympathetic skin response (SSR), and serum hormone (cortisol and norepinephrine) levels in healthy adults.
This study has been approved by Institutional Review Board at the Yonsei University Wonju Christian Hospital. Ethical considerations were addressed by explaining the research to the participants, and obtaining their written consent. In response to advertisements on bulletin boards and local hospital newspapers and to verbal requests, 151 volunteers applied for participation. At each session, if the participants refused to participate due to unwillingness to continue, heat and massage intervention was not carried out. Participants were excluded from the study if they have history of acute illness, malignancy, pregnancy, muscle disease, spinal posterior arch defect (e.g. spina bifida), previous operative history of lumbar and thoracic back region, and cognitive dysfunction. Demographic data were recorded for all subjects. In order to evaluate exclusion criteria, all subjects also underwent physical examination, electrocardiogram, radiography of the lumbar region, computed tomography of the lumbar region, and electromyogram (EMG).
The physiologic effects of heat and massage application vary, depending on tissue temperature, the duration of application, the rate of tissue temperature increase, the size of the application area, and location. To minimize this effect, the study was carried out when temperature was maintained 50-55℃ for 40 minutes, and automatically applied on T9-L3 spinal ganglia, in order to provide similar pressure on the same locations for each subject and session. To maintain constant temperature, temperature was constantly monitored with thermometer in the machine. To maintain constant massage area, the desired spinous process level was determined using the L5-S1 intervertebral space as an anatomical landmark. Furthermore, a line connecting the superior iliac crests located the L4 vertebra. Heat and massage applications were performed with Ceragem M3500 (CERAGEM Co. Ltd., Cheonan, Korea). The ergonomically designed guiding rail is attached to the horizontal mobile parts of the bed. This enables the internal projector to allow the massage effect. Heat was generated by the rolling jades (Fig. 1). It was carried out for 40 minutes daily, 5 days a week for 2 weeks, setting the temperature to 50-55℃ at the hospital care room. The following variables were recorded: SSR, HRV, and serum cortisol and plasma norepinephrine levels. Physical examination findings and vital signs were recorded at the beginning of the study and at 1 week after initial application. After 2 weeks of application, subjects again underwent physical examination, and HRV, SSR, serum cortisol, and plasma norepinephrine were measured. Two weeks after the cessation of application, and 4 weeks after the beginning of the study, these measures were repeated and recorded (Fig. 2). Intertester reliability on all dependent measures was established between clinical evaluators before the initiation of the study.
The response in the sympathetic and parasympathetic nervous system was estimated based on analysis of HRV, which refers to the beat-to-beat fluctuations in heart rate. The parasympathetic nervous system can quickly and finely adjust the time instant for the next heart beat, whereas sympathetic nervous system is a slower system for regulation of heart rate. This difference in activities creates variability within different frequency domains. The high frequency region (0.15-0.40 Hz) is mainly mediated by parasympathetic activity, whereas the low frequency region (0.04-0.15 Hz), standard deviation of RR interval (SDNN) and total power (TP) are mainly mediated by sympathetic nervous system in relation to stress. HRV was analyzed by power spectrum analysis of interbeat intervals. RR intervals were converted to a time series by cubic spline interpolation, followed by resampling at 2.4 Hz. HRV was determined using an SA-2000E (MEDICORE Co. Ltd., Seongnam, Korea). The following HRV indices were calculated: standard deviation of all normal RR intervals: the TP: the normalized low frequency power (LFP).
To obtain quantitative measurements of autonomic nervous system response, cortisol and norepinephrine levels were measured from a single 10 mL blood sample, collected into serum and plasma blood tubes. Participants were instructed to fast 2 hours prior to test, and blood sample were collected at same time of the day to minimize circadian and diurnal fluctuations in the biomarkers. Plasma was mixed and spun, and serum was allowed to clot for 30 minutes, and they were then centrifuged for 10 minutes. The quantitative measurement of cortisol and norepinephrine was performed using a commercially available competitive binding enzymimmunoassay according to the manufacturer's instructions. Data were collected three times at the beginning of study (pretest), 2 weeks after application, and 4 weeks after application (2 weeks after cessation of application) (Fig. 2). All analyses were conducted in the Clinical Chemical Laboratory at the University Hospital.
SSR test has proven to be useful in examining the function of sympathetic activities. As a reflex response, an abnormal SSR might be due to the impairment of afferent and efferent peripheral nervous system, and psychological dysfunction. Increased sympathetic activities such as high amplitude and decreased latency in pathologically stressful subjects have been observed.
Using a Viking IV (CareFusion, San Diego, CA, USA) instrument, SSR was monitored as an additional indicator of the change of the sympathetic nervous system reflecting stress. In order to analyze the neurocutaneous response in the frequency range, the filter setting was adjusted (0.1-20 Hz). To study the SSR, the subject was asked to relax and breath regularly for a short period of time, lying on a bed in a quiet and dimly lit room with an ambient temperature between 22 and 24℃. The skin underlying the recording sites was cleaned carefully with a dry cloth immediately prior to placing the surface electrodes, which were attached with sticking plaster. A round surface electrode 1 cm in diameter was attached 2.0 cm from the medial border of the left palm, and the reference electrode was attached at the opposite point on the dorsal side of the hand. Nerve stimulation was applied at maximum intensity on the proximal part of the volar wrist in the right median nerve distribution.
Repeated ANOVA test was used to analyze data between evaluation times. For data analysis before and after heat and massage application, paired-t tests were used. Analysis was performed using the SAS statistical software (Version 9. 1. 3) and statistical significance was accepted for p-values less than 0.05.
Among 151 initial subjects, three subjects were excluded because of arrhythmias found on the initial electrocardiogram (one subject with first-degree artrioventricular block and two subjects with premature ventricular complexes) and nine subjects dropped out during the study, yielding a total of 139 subjects who completed the study. Image analysis and EMG of the lumbar region were performed on all subjects for anatomical electrophysiological evaluation that include exclusion criteria, e.g. spina bifida, spinal tumor and muscular disease. There were no participants that had exclusion criteria; however, 54.3% had the following findings: intervertebral disc protrusion in 43 subjects (30.9%), degenerative spondylosis in 23 subjects (16.6%), spinal stenosis in seven subjects (5.1%), spondylolysis in seven subjects (5.1%), intervertebral disc calcification in four subjects (2.9%), and lumbar radiculopathy in fourteen subjects (10.1%) (Table 1).
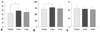
The average serum cortisol levels of the subjects were as follows: 9.54±2.01 pg/mL at baseline, 6.92±1.62 pg/mL after 2 weeks of therapy, and 8.29±2.11 pg/mL at 4 weeks after initiating the study. Serum cortisol levels after 2 weeks of treatment were significantly decreased compared with the baseline (p=0.003). Serum cortisol measured 4 weeks after beginning the study tended to be decreased compared with the baseline, but these results were not statistically significant (Fig. 3).
Average plasma norepinephrine levels of the subjects were as follows: 190.5±57.9 µg/dL at baseline, 170.9±93.3 µg/dL after 2 weeks of therapy, and 132.8±41.1 µg/dL at 4 weeks after beginning the study. These levels were significantly decreased at the 4-week time point compared with the baseline (p=0.010) (Fig. 3).
In the analysis of the power spectrum of the heart rate change index after 2 weeks of heat and massage application, TP and SDNN were significantly increased (p=0.014, 0.021), but there was no significant difference in the degree of change, SDNN, and TP after 4 weeks compared with the baseline. Normalized LFP tended to generally decrease, but the findings were not statistically significant (Fig. 4).
In the analysis of SSR, the latencies at 2 and 4 weeks post-treatment were 1,405.4±150.4 msec and 1,463.4±138.9 msec, respectively. Compared with the baseline of 1,231.0±221.3 msec, the latencies after 2 and 4 weeks were significantly increased (p=0.023, 0.012). Amplitude was 1,873.7±780.9 µV after 2 weeks and 1,598.2±936.2 µV after 4 weeks post-treatment, and was significantly decreased compared with the baseline of 2,108.4±1193.1 µV (p=0.008) (Fig. 5).
The aim of this study was to evaluate the effects of heat and massage application on autonomic responses, especially sympathetic activity, in relation to stress. The main findings in this study were that, after 2 weeks of heat and massage application, there was significant decrease in serum cortisol level, and there was significant decrease in serum norepinephrine level after 4 weeks, indicating an overall down regulation of sympathetic activity. In measurements of HRV using the power spectra, there was significant increase in TP and standard deviation, mainly mediated by sympathetic nervous system. Of autonomic nerve conduction measures, latency was significantly increased at 2 and 4 weeks, and amplitude was significantly decreased at 4 weeks. These findings would normally be interpreted as decreased down regulation of sympathetic activity, indicating reduced stress response. Ten subjects (7.2%) complained of a temporary increase in low back pain. The cause to induce temporary increased low back pain among our study population is likely multifactorial. First, although temperature and pressure were maintained to induce heat and massage effect, it is possible to induce tissue damage and consequent nociceptor activation. Second, it has been suggested that pre-existing low back discomfort could be extensive.
Activation of the autonomic nervous system, including hemodynamic, electrodermal, and hypothalamic-pituitary-adrenal system, influences metabolism. The sympathetic nervous system and stress contribute to catabolic activity and the consumption of energy, whereas the parasympathetic nervous system works for anabolic activity and the storage of energy. Stress can, therefore, cause catabolic activity. Our findings support the hypothesis that HRV, serum cortisol and norepinephrine levels, and SSR would be lowered after heat and massage application.
The autonomic nervous system is responsible for maintaining homeostasis against external conditions by controlling the activities of the viscera, blood vessels, and secretory glands.6 As the incidences of certain diseases such as diabetes, affecting the vascular system, are increasing, the awareness of their effects on the autonomic nervous system has been heightened. Various evaluation methods have been developed to examine the function of the autonomic nervous system, including measuring cardiovascular parameters related to posture and isometric exercises, measuring body surface temperature with infrared ray photography, evaluating the mobility of the gastrointestinal tract using certain isotopes, analyzing blood levels of autonomic neurotransmitters, testing electrophysiological parameters, examining the autonomic nervous system response to medication, quantitatively measuring sweating, and evaluating pupils and pudendal nerve responses.7-9 However, most of these evaluation methods have limited clinical applications because of difficulty in quantifying the results, lack of ability to reproduce the results, or they are very invasive.10 Heart rate is constantly changing to maintain homeostasis, and is determined by autonomic nervous system stimulation of the S-A node and spontaneous excitation of the S-A node.11,12 Since heart rate is controlled by the antagonistic work of sympathetic and parasympathetic nervous systems, the analysis of heart rate change can reflect the balance of each component of the autonomic nervous system.6,13-18 This analysis yields three points on the power spectrum: an ultra low frequency component of less than 0.05 Hz related to thermoregulation and the renin-angiotensin system, a low frequency component at around 0.1 Hz, and a high frequency component at around 0.25 Hz.10,12,17,19-24
In this study, analysis of the SDNN and TP indices were significantly increased after 2 weeks of heat and massage application, compared with the baseline, however, they tended to decrease 2 weeks after heat and massage application stopped, that is, 4 weeks after the study began. LFP was found to be generally decreased, but the findings were not statistically significant, implying that heat and massage applications lead to up-regulation of both autonomic activity. This notion is supported by the view that sympathetic and parasympathetic branches do not always act reciprocally, but may also act synergistically and complementarily.
The mechanism of the SSR has not yet been fully defined, but it has been demonstrated that electrical stimulation of peripheral nerves is transmitted to the lateral columns of the spinal cord through group II, and III fibers, that sweating is caused by transmission of impulses to the neuro-sweat gland junction through pre-ganglion and post-ganglion small diameter centrifugal fibers, and that temporary skin electric potential changes due to sweating can be recorded and are known as the SSR.13,25,26 Since Knezevic and Bajade27 outlined a method to record this skin response non-invasively, many researchers have been studying it. Aisen and Stallman28 reported that spinal cord injuries influence the SSR after examining it in patients with paraplegia, and Brown and Wang29 discovered that sensory motor areas, hypothalamus, and brainstem reticular system all exert an excitative effect on the SSR. The connections within the central nervous system for the provocation of the SSR consist of a multisynapse pathway, but the connection between the hypothalamus and the motor neurons of the spinal cord is unclear. The descending autonomic pathway in the brain stem and spinal cord is not limited to one area but is widely spread throughout.30,31 In 1993, Cheong, et al. reported that the latency of the SSR in spinal cord injury patients was longer and its amplitude was greater than that of normal controls. There was also a report that, in the case of patients with cerebrovascular accident, if the nerve stimulus area and the record area were different between right and left, the latency was longer than when they were same.32 Furthermore, in patients with anxiety and multiple sclerosis, 94.2% of SSR were found to be abnormal, and the ratio of abnormal findings was higher than that of any other evoked potential test.30 Exposure to continuous stress causes functional changes in the autonomic nervous system, and the SSR intensity in electric nerve conduction tests is generally recognized to increase such as increased amplitude and decreased latency.33 In SSR measurements in this study, the latency was found to be significantly increased after 2 and 4 weeks of heat and massage application, and the amplitude was significantly decreased compared with the baseline after 4 weeks of application, indicating overall decrease of sympathetic activity.
Catecholamine release in the bloodstream is increased under a variety of conditions, including stresses, hypoglycemia, hypovolemia secondary to bleeding, and hypoxemia. Furthermore, it is very sensitive to changes in the blood sugar level, posture, and activity level, and also shows episodic daily changes. Cortisol is secreted from the adrenal cortex, in response to ACTH release by the pituitary gland, and more than 90% of it in the blood are conjugated to proteins and excreted in urine after being metabolized in the adrenal gland, liver, and kidneys. Together with aldosterone, cortisol is an important hormone in the adrenal cortex and an important part of the stress response.31 Norepinephrine secreted by sympathetic nerve endings binds to receptors on neighboring organs and influences their function, and then undergoes re-uptake at the sympathetic nerve endings. Epinephrine and norepinephrine secreted by the adrenal medulla enter the bloodstream and exert their effects on organs farther away. These hormones work by stimulating the α and β receptors, each having a specific effect at each receptor type. Adrenal medulla secretes epinephrine and norepinephrine at the ratio of 9 : 1, measured in the renal vein. In the capillaries, this ratio decreases to about 1 : 4. This is due to the fact that epinephrine is made only in the adrenal medulla and the clearance of epinephrine and norepinephrine is almost the same. Epinephrine secretion can, therefore, be used as a reflex of sympathetic nerve function, as well as adrenal medulla function. In clinical diagnostic testing, blood catecholamine levels can be used to diagnose pheochromocytoma and neuroblastoma. In addition, catecholamine increases can be seen in essential hypertension, renal hypertension, malignant hypertension, and congestive heart failure, but catecholamine monitoring in these diseases is not diagnostically useful. Under stress, catecholamine levels can also be increased.34 In this study, plasma norepinephrine after 2 weeks of heat and massage applications was found to decrease at 2 and 4 weeks compared with the baseline. Serum cortisol levels at 2 weeks and plasma norepinephrine levels at 4 weeks were also significantly decreased. These findings indicate decreased activity of the Hypothalamic-Pituitary-Adrenal axis. These implicate that heat and massage application contribute to decrease catabolic activity.
In conclusion, we objectively evaluated that the effect of heat and massage application provide relaxation in the autonomic nervous system through comparison analysis of data obtained before and after heat and massage application. Despite the impressive results of heat and massage application found in this study, cautious interpretation of study findings is warranted because of some limitations, such as experimental design without a control condition, study design without a self-report measure of stress to have clinical application. The majority of the sample was female so there was some limitation to generalize to male population. These results reflect the effects of heat with mechanical massage but cannot be compared directly to results of manually-derived massage. Given limitations noted in this study, future studies with blinding and randomized allocation of study subjects, a controlled group, and the effect of heat vs. massage isolated study are important to validate the effectiveness of heat and massage application.
Figures and Tables
 | Fig. 1The picture of treatment device (Ceragem M3500). The internal projector moves along the natural back line. |
 | Fig. 3Plasma cortisol (upper panel) and norepinephrine (lower panel) variability according to time. *p<0.05. |
References
1. Min SK. Modern Psychiatry. 1999. 4th ed. Seoul: Ilchokak.
2. Ahn PJ, Chang BH, Choi JW. Evaluation of wear sensation with garment of Far Infrared ray radiation fabric at office in summer. J Korean Soc Living Environ Syst. 1997. 4:47–56.
3. Moraska A, Pollini RA, Boulanger K, Brooks MZ, Teitlebaum L. Physiological adjustments to stress measures following massage therapy: a review of the literature. Evid Based Complement Alternat Med. 2010. 7:409–418.

4. Diego MA, Field T. Moderate pressure massage elicits a parasympathetic nervous system response. Int J Neurosci. 2009. 119:630–638.

5. Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998. 23:819–835.

6. Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension. 1995. 25:1276–1286.

7. Kim SH, Kim HT, Kim JH, Chung KC, Kim MH. The relationship between hemispheric lesion and autonomic function by using beat-to-beat heart rate variation. J Korean Neurol Assoc. 1988. 6:49–54.
8. Shin JB, Chon JS, Ha KH, Chun SI. The habituation phenomenon of sympathetic skin response. J Korean Acad Rehabil Med. 1991. 15:40–46.
9. Korpelainen JT, Sotaniemi KA, Suominen K, Tolonen U, Myllylä VV. Cardiovascular autonomic reflexes in brain infarction. Stroke. 1994. 25:787–792.

10. Pieper SJ, Hammill SC. Heart rate variability: technique and investigational applications in cardiovascular medicine. Mayo Clin Proc. 1995. 70:955–964.

12. Kamath MV, Fallen EL. Power spectral analysis of heart rate variability: a noninvasive signature of cardiac autonomic function. Crit Rev Biomed Eng. 1993. 21:245–311.
13. Montagna P, Liguori R, Zappia M. Sympathetic skin response. J Neurol Neurosurg Psychiatry. 1985. 48:489–490.

14. Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation. 1994. 90:1826–1831.

15. Kamalesh M, Burger AJ, Kumar S, Nesto R. Reproducibility of time and frequency domain analysis of heart rate variability in patients with chronic stable angina. Pacing Clin Electrophysiol. 1995. 18:1991–1994.

16. Vardas P, Kochiadakis G, Orfanakis A, Kalaitzakis M, Manios E. Intraindividual reproducibility of heart rate variability before and during postural tilt in patients with syncope of unknown origin. Pacing Clin Electrophysiol. 1994. 17:2207–2210.

17. Malliani A, Lombardi F, Pagani M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br Heart J. 1994. 71:1–2.

18. Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J. 1994. 127:1376–1381.

19. Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981. 213:220–222.

20. Burke JH, Goldberger JJ, Ehlert FA, Kruse JT, Parker MA, Kadish AH. Gender differences in heart rate before and after autonomic blockade: evidence against an intrinsic gender effect. Am J Med. 1996. 100:537–543.

21. Hedman AE, Tahvanainen KU, Hartikainen JE, Hakumäki MO. Effect of sympathetic modulation and sympatho-vagal interaction on heart rate variability in anaesthetized dogs. Acta Physiol Scand. 1995. 155:205–214.

22. Jaffe RS, Fung DL, Behrman KH. Optimal frequency ranges for extracting information on autonomic activity from the heart rate spectrogram. J Auton Nerv Syst. 1994. 46:37–46.

23. Takalo R, Korhonen I, Turjanmaa V, Majahalme S, Tuomisto M, Uusitalo A. Short-term variability of blood pressure and heart rate in borderline and mildly hypertensive subjects. Hypertension. 1994. 23:18–24.

24. Rollins MD, Jenkins JG, Carson DJ, McClure BG, Mitchell RH, Imam SZ. Power spectral analysis of the electrocardiogram in diabetic children. Diabetologia. 1992. 35:452–455.

25. Day TJ, Offerman D, Bajada S. Peripheral sympathetic conduction velocity calculated from surface potentials. Clin Exp Neurol. 1986. 22:41–46.

26. Fagius J. Aspects of autonomic neurophysiology in diabetic polyneuropathy: a brief review. Diabet Med. 1991. 8(Spec No):S58–S62.

27. Knezevic W, Bajada S. Peripheral autonomic surface potential. A quantitative technique for recording sympathetic conduction in man. J Neurol Sci. 1985. 67:239–251.
28. Aisen ML, Stallman J. The sympathetic skin response in quadriplegia. J Neurol Rehabil. 1955. 9:1–5.

29. Brown VM, Wang GH. Changes in galvanic skin reflex after acute spinal transection in normal and decerebrate cats. J Neurophysiol. 1956. 19:446–451.

30. Elie B, Louboutin JP. Sympathetic skin response (SSR) is abnormal in multiple sclerosis. Muscle Nerve. 1995. 18:185–189.

31. Shahani BT, Halperin JJ, Boulu P, Cohen J. Sympathetic skin response--a method of assessing unmyelinated axon dysfunction in peripheral neuropathies. J Neurol Neurosurg Psychiatry. 1984. 47:536–542.

32. Zimmermann KP, Monga TN, Darouiche RO, Lawrence SA. Post-stroke autonomic nervous system function: palmar sympathetic skin responses thirty or more days after cerebrovascular accident. Arch Phys Med Rehabil. 1995. 76:250–256.

33. Mohan A, Sharma R, Bijlani RL. Effect of meditation on stress-induced changes in cognitive functions. J Altern Complement Med. 2011. 17:207–212.

34. Lee KN. Clinical Pathology file. 1993. 2nd ed. Seoul: Korea Clinical Laboratories.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download