Abstract
Purpose
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is an inflammatory enzyme expressed in atherosclerotic plaques. We investigated the association of circulating Lp-PLA2 with characteristics of vulnerable coronary atherosclerotic plaques.
Materials and Methods
We recruited 113 patients with either unstable angina (UA, n=59) and stable angina (SA, n=54) by coronary angiography. Thirty-six healthy subjects served as controls. Intravascular ultrasound (IVUS) was used to evaluate the characteristics of coronary atherosclerotic plaque, and serum Lp-PLA2 concentration was measured as well.
Results
Lp-PLA2 concentration was significantly higher in both UA and SA patients [(396±36) µg/L and (321±39) µg/L, respectively] compared with the controls [(127±49) µg/L, p<0.01], and higher in UA than SA group. IVUS findings showed that remodeling index (RI) (0.91±0.15 vs. 0.85±0.11, p=0.005) and eccentricity index (EI) (0.73±0.16 vs. 0.65±0.22, p=0.039) were larger in UA than in SA group, and fibrous caps were thicker in SA than UA group [(0.91±0.23) mm vs. (0.63±0.21) mm, p=0.032]. Moreover, Lp-PLA2 correlated positively with EI (r=0.439, p<0.01) and RI (r=0.592, p<0.05) in UA group. There was an inverse relationship between Lp-PLA2 and fibrous cap thickness in both UA (r=-0.587, p<0.001) and SA (r=-0.318, p<0.05) groups. The independent risk factors in UA group were Lp-PLA2 (OR=1.055, 95% CI: 1.03-1.08, p=0.013), LDL-cholesterol (OR=0.032, 95% CI: 0.00-0.05, p=0.041) and fibrous cap thickness (OR=0.008, 95% CI: 0.00-0.45, p=0.019). Lp-PLA2 was strongly associated with both EI and fibrous cap thickness in both groups.
Accumulating evidence indicates that inflammation is recognized to play an important role in atherosclerosis, atherosclerotic plaque progression, or even predisposing vulnerable plaque to rupture.1 Peripheral blood biomarkers of inflammation have been associated with incident and recurrent cardiac events. Inflammatory cells, cytokines and other biomolecules are implicated in these processes, and have, therefore, been investigated as potential markers of atherosclerotic plaque progression and cardiovascular disease risk. The levels of plasma markers of inflammation such as C-reactive protein are elevated in coronary heart disease, especially acute coronary syndrome.2
Recent studies show that lipoprotein-associated phospholipase A2 (Lp-PLA2) is an independent predictor of coronary heart disease,3 expressed by inflammatory cells in atherosclerotic plaques which hydrolyses oxidised phospholipids to yield pro-inflammatory products implicated in endothelial dysfunction, plaque inflammation, and formation of necrotic core in plaque, thus postulating a link between oxidative modification of low-density lipoprotein (LDL)-cholesterol and development of inflammatory responses in the arterial intima.4,5 Two thirds of plasma Lp-PLA2 are bound to LDL-cholesterol molecules, whereas the remainder is distributed between LDL-cholesterol and very low density lipoproteins.5-7 Lp-PLA2 is expressed abundantly in the necrotic core of atherosclerosis plaque, and its' enzymatic products participate in the process of inflammation and cell death, rendering plaque vulnerable to rupture.8-10
Considering a potential need for inflammatory biomarkers, we aimed to explore the association of Lp-PLA2 with characteristics of vulnerable coronary atherosclerotic plaques in patients with coronary heart disease by examining the relationships between serum levels of Lp-PLA2 and the parameters obtained by intravascular ultrasound (IVUS).
A total of 113 patients were recruited, and all patients were approved by the Ethics Committee of Shandong University. The range of age was 35-81 years (mean age 58.2±10.6 years); 58 were male and 55 were female. These patients were diagnosed as stable angina (SA, 54 cases) or unstable angina (UA, 59 cases), showing a segmental stenosis with 20% to 70% lumen diameter reduction in one major coronary artery and at least 2.25 mm in diameter on coronary angiography. IVUS examinations were performed as well during the course of coronary catheterization. In addition, 36 healthy subjects with a mean age of (57.9±10.9) (37-75) years served as controls. Written informed consent was obtained from patients and controls, and the research protocols were approved by the Institutional Board of the Second Hospital of Shandong University.
SA was defined as no changes in angina symptoms in terms of frequency, duration, or intensity in the preceding four weeks with normal cardiac enzymes and UA was defined as accelerated angina, new-onset severe angina, or angina at rest within four weeks, with electrocardiographic changes of ischemia (ST-segment depression or elevation of at least 1 mm) or a less than two-fold increase in levels of cardiac enzymes or abnormal cardiac troponin I and/or T. For all study groups, the exclusion criteria were acute myocardial infarction, chronic total occlusions, severe angular, calcific or diffuse lesions in the culprit artery, acute infection or autoimmune diseases, previous percutaneous coronary interventions or coronary artery bypass graft, valvular heart disease, congestive heart failure (LVEF<30%), malignant tumors, and severe liver disease (plasma alanine aminotransferase level >120U/L). Major Adverse Cardiovascular Events (MACE) including acute ST-elevation myocardial infarction, revascularization, or even cardiovascular death were followed for about 18 months. The patient population was stratified according to ACS status.11
IVUS examination (with a 2.9F, 40 MHz mechanical transducer, Boston Scientific Galaxy I, Minneapolis, MN, USA) was performed during angiography. The IVUS catheter was advanced distal to the target lesion, and imaging was recorded retrograde to the aorto-ostial junction at an automatic pullback speed of 0.5 mm/s. In addition, the culprit lesion was discussed with manual interrogation. Quantitative IVUS analysis was performed by use of planimetry software. Offline qualitative and quantitative analyses of IVUS images were performed by two independent experienced IVUS investigators, blinded to clinical data, following the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition,12 Measurement and Reporting of IVUS Studies.
The proximal or distal reference sites and the culprit lesion site were identified in the target vessel. Proximal and distal references were the single slices with largest lumen and smallest plaque burden within 10 mm proximally and distally, but before any large side branch and the culprit lesion site was defined as the image slice with smallest lumen cross sectional area (CSA). The external elastic membrane (EEM) CSA was measured by tracing the leading edge of the media-adventitia boundary and lumen CSA was determined by tracing the boundary between the lumen and the intimal leading edge. Plaque area (EEM CSA-lumen CSA), plaque burden (plaque area/EEM CSA), and lumen area stenosis [(reference lumen CSA-minimum lumen CSA)/reference lumen CSA] was calculated.
The maximal and minimal thickness of the vessel wall was measured, and the atheroma eccentricity index (EI) [(maximum plaque thickness-minimum plaque thickness)/maximum plaque thickness] was then calculated at the culprit lesion site. EI <0.5 or ≥0.5 was respectively defined as concentric or eccentric plaque. Eccentric plaque seems to be more vulnerable than concentric because eccentric plaque is likely to rupture by various intra-luminal stresses. Coronary artery remodeling index (RI) was usually calculated as: the EEM CSA at the minimal lesion site/average EEM CSA at the proximal and distal reference sites. Positive remodeling, intermediate remodeling and negative remodeling were described as RI >1.05, 0.95-1.05, and <0.95, respectively.
The atherosclerotic lesion with positive remodeling has usually a larger lipid core, and is more vulnerable. Plaques were classified into soft, fibrous, calcific and mixed plaques seperately according to the characteristics of the IVUS images.12 The echolucent zone in a plaque on IVUS may demonstrate a lipid-rich core. Soft plaque is considered as a vulnerable plaque due to fibrous cap unformed. Vulnerability index here includes all the variables analyzed by IVUS, especially RI, EI, positive remodeling, soft plaque and fibrous cap thickness. Large lipid core, thin fibrous cap, large intraplaque hemorrhage might imply highly unstable coronary events.13
Biochemical analyses was performed in blinded arrangement. Fasting blood samples were centrifuged at 1,500×g for 10 minutes at 4℃, then stored at -80℃ before use. Plasma cholesterol, triglyceride, high-density lipoprotein (HDL), low-density lipoprotein (LDL), fasting blood glucose, creatinine, uric acid and fibrinogen were tested by a biochemical analyser (Hitachi-7600, Tokyo, Japan). The intra- and inter-assay coefficients of variation were <5%. Serum Lp-PLA2 was measured by a colorimetric method with an intra-assay precision of 1.7% and inter-assay precision of 4.8%. Details regarding other biomarkers are provided in the online data supplement.
Statistical analysis was performed using SPSS software (version 13.0, SPSS Inc., Chicago, IL, USA). Data were reported as mean values±one SD and first tested for normality of distribution by the Shapiro-Wilk test. Comparisons of categorical and continuous variables were performed by chi-square or Fisher's exact test. Relationship among variables was appropriately tested using Pearson correlation test or Spearman rank order correlation test. Cardiovascular risk factors of unstable angina by multiple logistic regression analysis and the association between Lp-PLA2 concentration and parameters obtained by IVUS were tested by linear regression analysis. A two-tailed p-value <0.05 was considered to be statistically significant.
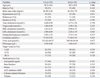
The baseline characteristics of UA and SA patients are presented in Table 1. Three experienced observers reviewed patients' clinical and angiographic data to ascertain degree of coronary stenosis and angina status, respectively. No significant differences exist in the use of angiotension-converting enzyme inhibitors/angiotensin-receptor blockers, beta-blockers, calcium channel blockers, statins, anti-platelet agents and nitrates between the two groups.
Baseline concentrations of serum Lp-PLA2 were significantly higher in UA and SA patients [(396±36) µg/L and (321±39) µg/L, respectively] as compared with that of controls [(127±49) µg/L, p<0.01]. And serum concentrations of Lp-PLA2 were higher in UA group than in SA group (Fig. 1).
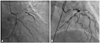
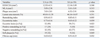
At the culprit lesion site, minimum lumen area, EEM CSA, and lumen area stenosis had no significant differences between UA and SA groups. Parameters of proximal and distal reference vessels were similar between the two groups. However, plaque area [(7.83±2.81) mm2 vs. (6.52±2.91) mm2, p=0.026], RI (0.91±0.15 vs. 0.85±0.11, p=0.005) and EI (0.73±0.16 vs. 0.65±0. 22, p=0.039) were larger in UA group than in SA group, whereas fibrous caps were thicker in SA group than in UA group [(0.91±0.23) mm vs. (0.63±0.21) mm, p=0.032]. Moreover, positive remodeling was more frequent in UA group than in the SA group (29% vs. 10%, p=0.021), and negative remodeling was less in UA group than in SA group (55% vs. 77%, p=0.016). Parameters from IVUS findings are shown in Table 2. Part of results by coronary angiography and IVUS are showed in Fig. 2 and 3.
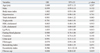
Multiple logistic regression analysisshowed that the independent cardiovascular risk factors of the patients with unstable angina were serum levels of Lp-PLA2 (OR=1.055, 95% CI: 1.03-1.08, p=0.013), LDL-cholesterol (OR=0.032, 95% CI: 0.00-0.05, p=0.041) and fibrous cap thickness (OR=0.008, 95% CI: 0.00-0.45, p=0.019). Although there was no signaficant difference between the two groups including age, gender, BMI, hypertension, total cholesterol, triglyceride, HDL-cholesterol, fasting blood glucose, creatinine, urine acid and fibrinogen they were conventional risk predictors (Table 3).
Serum level of Lp-PLA2 correlated positively with eccentricity index (r=0.439, p<0.01) (Fig. 4A) and remodeling index (r=0.592, p<0.05) (Fig. 4B) in UA group. There was an inverse relationship between the serum level of Lp-PLA2 and the fibrous cap thickness in both UA (r=-0.587, p<0.001) and SA groups (r=-0.318, p<0.05). Serum Lp-PLA2 did not correlate with other IVUS parameters in either group.
Serum level of Lp-PLA2 was strongly associated with both eccentricity index and fibrous cap thickness in both UA and SA groups. And fibrous cap thickness, the first of the three variables in either UA group (β=-0.408, p<0.001) or SA group (β=-0.337, p=0.008) was associated negatively with Lp-PLA2. However, remodeling index was associated with Lp-PLA2 only in UA group (β=0.356, p=0.001), but not in SA group (β=0.236, p=0.072) (Table 4).
According to the local and systemic inflammatory responses observed throughout the spectrum of atherosclerotic disease, from initial lesion formation to plaque destabilization or rupture, inflammation plays a primary role in the progression of atherosclerosis.14 The key role of inflammation is evidenced by numerous epidemiology studies to indicate the existence of inflammatory cells in the cap of atherosclerotic plaques3,10 and an association between circulating inflammatory markers (e.g., C-reactive protein, interleukin-6).2,15
Lp-PLA2, known as platelet-activating factor acetylhydrolase, is a 50 kDa, Ca2+ independent enzyme associated with LDL-cholesterol. The enzyme is a member of a growing family of phospholipases A2 and secreted mainly by macrophages/monocytes, mast cells, and T lymphocytes.5,6,16 Kolodgie, et al.10 found the key role of macrophages in fibrous cap thickness and necrotic core expansion, along with relationship between macrophages and Lp-PLA2 expression in the fibrous cap region, which indicated that Lp-PLA2 is involved in plaque vulnerability, particularly in the progression spanning from thin-cap fibroatheromas to plaque rupture. Rosenson17 demonstrated that circulating Lp-PLA2 is a novel inflammatory biomarker and additive to traditional risk factors for atherogenesis. Rosenson, however, measured only circulating levels of Lp-PLA2 (Lp-PLA2 mass), but not Lp-PLA2 activity, because previously studies4,18 demonstrated that Lp-PLA2 activity and mass are positively associated with each other.
In agreement with the findings by Kolodgie, et al.10 and Packard, et al.3 our results showed that Lp-PLA2 concentration was significantly increased in UA and SA patients, and that it was higher in UA group, suggesting that higher level of Lp-PLA2 is correlated with some morphologic variables indicative of vulnerable plaques, and that larger atherosclerotic burden may be useful for the recognition of high-risk patients. Major novelties of the current study include the correlation of higher concentration of Lp-PLA2 with the increased remodeling index and eccentricity index in UA patients, evidenced by IVUS in vivo, but not in SA patients, and an inverse relationship between the concentration of Lp-PLA2 and the fibrous cap thickness in both groups. Data from IVUS findings showed that plaque area, remodeling index, and eccentricity index were larger in UA group than those in SA group. Moreover, fibrous caps were thicker in SA patients than in UA patients. Therefore, positive remodeling was more frequent in UA group than in SA group, and negative remodeling was less in UA patients. The above observations together show that higher concentration of Lp-PLA2 imply more serious coronary atherosclerosis and may be prone to high-risk or vulnerable coronary plaques in angina patients, especially in UA patients. These data are in accordance with the histopathological findings by Kolodgie, et al.10 that macrophages and Lp-PLA2 expression in the fibrous cap region may indicate that Lp-PLA2 is involved in plaque vulnerability, particularly in the progression from thin-cap fibroatheromas to plaque rupture. Our findings suggested that Lp-PLA2 was released into circulation in the process of atherosclerosis and involved in the activity and vulnerability of atherosclerotic plaque.
The vulnerable plaque, rather than the degree of luminal narrowing, is a major factor contributing to acute coronary syndrome, which has been clarified by pathologic studies.19 The main components of most vulnerable plaques are usually thin fibrous cap, large lipid pool and active inflammation.20 Larger plaque area and positive remodeling are seen frequently in acute coronary syndrome, on the contary, smaller plaque area and negative remodeling are often linked to SA.20,21 Our study indicated that UA patients had higher RI, eccentricity index, larger plaque area and plaque burden, and more positive remodeling and eccentric plaques, indicating a close relationship between angina status and the plaque morphology. In addition, these findings validate the diagnostic value of Lp-PLA2 as a specific biomarker to differentiate unstable angina patients with a high risk of vulnerable coronary atherosclerotic plaques from stable angina and provide further evidence for its potential applicability in clinical practice.
In summary, the present findings indicate an association of Lp-PLA2 expression and advanced ruptured and rupture-prone lesions as thin-cap fibroatheromas. We obtained the parameters of coronary atherosclerotic plaque by IVUS in vivo and serum levels of Lp-PLA2. The results can reflect the relation between Lp-PLA2 and eccentricity index and fibrous cap thickness. Although the findings from the present study are intriguing and suggest biologic plausibility and specificity to vascular inflammation, a causal role of Lp-PLA2 in the progression of atherosclerosis and plaque vulnerability will likely requires further research.
This was an exploratory study that used novel imaging IVUS modalities to assess plaque composition and characteristic. Lp-PLA2 activity was not determined, and the clinical relevance of plaque stability observed with serum Lp-PLA2 requires a larger sample size. This study focused on a small segment of the coronary arterial tree and limited, and it might not fully represent disease progression elsewhere. Finally, the approach undertaken in this and other IVUS trials did not assess changes in the precise plaque phenotype that may portend clinical risk.
Figures and Tables
Fig. 1
Serum concentions of Lp-PLA2 in control subjects, SA group, and UA group. *p<0.01, compared with control. †p=0.014, compared with SA group. Lp-PLA2, lipoprotein-associated phospholipase A2; SA, stable angina; UA, unstable angina.

Fig. 3
IVUS image of control shows one patient with chest pain and no stenosis found by coronary angiography; compared with SA group, IVUS image of UA group shows vulnerable plaque. IVUS, intravascular ultrasound; SA, stable angina; UA, unstable angina.

Fig. 4
Correlation of serum level of Lp-PLA2 with eccentricity index (A), remodeling index (B) and fibrous cap (C) in UA and SA groups. Solid line, fit line for UA group; dotted line, fit line for SA group. UA, unstable angina; SA, stable angina.

ACKNOWLEDGEMENTS
This study was supported by a grant from the Key Technologies Research and Development Program of Shandong Province (2006GG2202016) in China. This work was also supported by The National Basic Research Program (973 Program) (2010CB7326050); The National Science & Technology Pillar Program during the Eleventh Five-Year Plan Period; Establishment of an integrated system for CHD prevention and treatment (2006BAI01A02). The authors are grateful for the editing of the manuscript by Professor Patricia Lounsbury, FAACVPR from the Iowa University, Heart & Vascular Care, USA.
References
1. Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004. 66:802–813.

2. Genest J. C-reactive protein: risk factor, biomarker and/or therapeutic target? Can J Cardiol. 2010. 26:Suppl A. 41A–44A.

3. Packard CJ, O'Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, et al. West of Scotland Coronary Prevention Study Group. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. N Engl J Med. 2000. 343:1148–1155.

4. Lp-PLA(2) Studies Collaboration. Thompson A, Gao P, Orfei L, Watson S, Di Angelantonio E, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010. 375:1536–1544.

5. Tellis CC, Tselepis AD. The role of lipoprotein-associated phospholipase A2 in atherosclerosis may depend on its lipoprotein carrier in plasma. Biochim Biophys Acta. 2009. 1791:327–338.

6. Asano K, Okamoto S, Fukunaga K, Shiomi T, Mori T, Iwata M, et al. Cellular source(s) of platelet-activating-factor acetylhydrolase activity in plasma. Biochem Biophys Res Commun. 1999. 261:511–514.

7. Stafforini DM, Elstad MR, McIntyre TM, Zimmerman GA, Prescott SM. Human macrophages secret platelet-activating factor acetylhydrolase. J Biol Chem. 1990. 265:9682–9687.

8. Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol. 2005. 25:923–931.
9. Häkkinen T, Luoma JS, Hiltunen MO, Macphee CH, Milliner KJ, Patel L, et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999. 19:2909–2917.
10. Kolodgie FD, Burke AP, Skorija KS, Ladich E, Kutys R, Makuria AT, et al. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2006. 26:2523–2529.

11. Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003. 108:1772–1778.
12. Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001. 37:1478–1492.

13. Tang D, Yang C, Zheng J, Woodard PK, Saffitz JE, Petruccelli JD, et al. Local maximal stress hypothesis and computational plaque vulnerability index for atherosclerotic plaque assessment. Ann Biomed Eng. 2005. 33:1789–1801.

14. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005. 352:1685–1695.

15. Anderson JL. Lipoprotein-associated phospholipase A2: an independent predictor of coronary artery disease events in primary and secondary prevention. Am J Cardiol. 2008. 101:23F–33F.

16. Reddy KJ, Singh M, Bangit JR, Batsell RR. The role of lipoprotein-associated phospholipase A2 on cardiovascular disease risk assessment and plaque rupture: a clinical review. J Clin Lipidol. 2009. 3:85–93.

17. Rosenson RS. Lp-PLA(2) and risk of atherosclerotic vascular disease. Lancet. 2010. 375:1498–1500.

18. Brilakis ES, Khera A, McGuire DK, See R, Banerjee S, Murphy SA, et al. Influence of race and sex on lipoprotein-associated phospholipase A2 levels: observations from the Dallas Heart Study. Atherosclerosis. 2008. 199:110–115.

19. Virmani R, Burke AP, Kolodgie FD, Farb A. Pathology of the thin-cap fibroatheroma: a type of vulnerable plaque. J Interv Cardiol. 2003. 16:267–272.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download