Abstract
Purpose
Our study is performed to find out clinicopathlogic and immunohistochemical (IHC) characteristics of triple negative invasive lobular carcinoma (ILC), as has been demonstrated in their invasive ductal counterparts.
Materials and Methods
Retrospective analysis of variable clinicopathlogic parameters and IHC stains for androgen receptor, estrogen receptor, progesterone receptor, p53, c-kit, galectin-3, cytokeratin 5 (CK5), CK5/6, vimentin, E-cadherin, epidermal growth factor receptor, and HER2 were performed in 117 cases of ILC.
Results
Eight cases (6.8%) were triple negative carcinoma (TNC), which showed higher incidence of high histologic grade than non-TNC (p = 0.019). Galectin-3 was expressed with higher incidence in tumor cells of TNC (62.5%) than those of non-TNC (7.3%) (p = 0.000). In contrast, galectin-3 was expressed with higher incidence in stromal cells of non-TNC (53.2%) than those of TNC (12.5%) (p = 0.029). CK5 and CK5/6 were not expressed in all ILCs.
Conclusion
TNC in ILC showed distinct clinicopathologic and IHC characteristics such as higher histologic grade and increased expression of galectin-3, compared to non-TNC in ILC. TNC in ILC was less frequent and did not show CK5 and CK5/6 expression when compared to TNC in invasive ductal carcinoma.
Invasive lobular carcinoma (ILC) is a distinctive subtype of invasive breast carcinoma and comprise about 5-15% of all carcinoma of the breast.1,2 Substantial studies have shown that ILC has a distinctive characteristic that differs from invasive ductal carcinoma (IDC) not only in histologic and clinical characteristics,3,4 but also in gene analysis profile,5 immunophenotype3,6-8 and response to systemic therapy.6-8 ILC demonstrates high incidence of older age, larger tumor size, low histologic grade, less lymphatic invasion, loss of E-cadherin expression and frequently expression of hormone receptors.2 ILC shows a high rate of bilateral disease, multiple metastasis and unique patterns of metastasis compared with IDC.4,9,10
In gene expression profiling analysis, breast cancer has been classified into luminal A, luminal B, HER2 overexpressiong, normal breast-like, and basal-like type.11 About 80-90% of triple negative carcinoma (TNC) overlaps with basal-like breast cancer (BLBC) according to DNA microarray and immunohistochemical (IHC) study and have clinical behavior similar to BLBC.12 TNC has no effective modalities because of TNC is estrogen receptor (ER) and progesterone receptor (PR) negative for hormone treatment, and HER2 negative for trastuzumab treatment. TNC (ER-, PR-, HER2-) was used as a surrogate for BLBC because most BLBC did not express ER, PR, and HER2 and it has the advantage that those three stains have already been used routinely in clinical work-up of breast cancer.13,14 However, the overlap between BLBC and TNC is not complete.15 BLBC classified by microarray DNA has revealed that 15-54% of them express at least one of three markers.14,16-19 In addition, TNC encompasses another molecular subgroup, namely normal-like breast cancer which has been shown to have slightly better prognosis than BLBC11,19 and to demonstrate no response to neoadjuvant chemotherapy.18
Most of previous studies on BLBCs have been performed on data sets and were predominantly composed of IDC. As least 85% of the cases from which the intrinsic gene set was derived was ductal type carcinoma.11,19-21 Although previous reports have shown that small subsets of ILC express basal cytokeratins (CKs),22-24 there have been no studies that systematically evaluated the incidence and significance of the TNC in ILC.
The purpose of our study is to investigate clinicopathologic and IHC characteristics of TNC in ILC.
From the files of the Department of Pathology in Severance Hospital, tissue samples from female patients with ILC were retrieved. The study was approved by the Institutional Review Board. All patients were diagnosed as having ILC by pathologists. All tissues were fixed in 10% buffered formalin and embedded in paraffin. All archival hematoxylin and eosin (H&E)-stained slides for each case were reviewed by 2 pathologists (Koo JS and Jung WH). Cases of mixed invasive lobular and ductal carcinoma were excluded. ILC of classic type was defined as small, relatively uniform carcinoma cells which invaded the stroma singly and in a single file pattern. And ILC of pleomorphic type was defined as carcinoma cells with larger and more nuclear variation than those seen in classic type ILC. The histological grade was assessed using Nottingham grading system,25 and nuclear grade was evaluated according to modified Black's nuclear grade (1 = low grade, 2 = intermediate grade, and 3 = high grade).26 Histologic parameters were evaluated from the H&E-stained slides. Clinicopathologic parameters evaluated in each tumor included patient's age at initial diagnosis, lymph node status, histological subtype, histologic grade, nuclear grade, tumor recurrence, time to recurrence, distant metastasis, and survival time.
On the H&E-stained slide of the tumor, a representative area was selected and corresponding spot was marked on the surface of the paraffin block. Using a biopsy needle, the selected area was punched out and the 3 mm tissue core was placed in a 5 × 6 recipient block. At least, more than two tissue cores were extracted to minimize extraction bias. Each separate tissue core was assigned with a unique tissue microarray location number that was linked to database including other clinical-pathologic data.
The antibodies used for immunohistochemistry in this study are shown in Table 1. All immunostainings were performed using formalin-fixed, paraffin-embedded tissue sections. 5 µm thick sections were obtained with a microtome, transferred into adhesive slides, and dried at 62℃ for 30 min. After incubation with primary antibodies, immunodetection was performed with biotinylated antimouse immunoglobulin, followed by peroxidase-labeled streptavidin using a labeled streptavidin biotin kit with 3,3'-diaminobenzidine chromogen as substrate. Optimal incubation time and concentration of the primary antibody were determined via serial dilution for each IHC assay with an identically fixed and embedded tissue block. Slides were counterstained with Harris hematoxylin. The staining was interpreted by two pathologists on a multiview microscope.
All IHC markers were assessed by light microscopy. Scoring of immunostained slides was done according to the percentage of tumor cells exhibiting nuclear [androgen receptor (AR), ER, PR, and p53], nuclear and cytoplasmic (c-kit, galectin-3), cytoplasmic (CK5, CK5/6, and vimentin), and membrane [E-cadherin, epidermal growth factor receptor (EGFR), and HER2] staining. The ER and PR immunohistochemistry was scored using the so-called 'Allred Score'.27 Briefly, a proportion score was assigned, representing the estimated proportion of positive staining tumor cells (0 = none; 1 < 1/100; 2 = 1/100 to < 1/10; 3 = 1/10 to < 1/3; 4 = 1/3-2/3; 5 = > 2/3). Average estimated intensity of staining in positive cells was assigned with an intensity score (0 = none; 1 = weak; 2 = intermediate; 3 = strong). Proportion score and intensity score were added to obtain a total score that ranged from 0-8. The score of 0 to 2 was considered negative, and the score of 3 to 8 was considered positive. HER2 staining was scored according to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guideline28 using the following categories: 0, no immunostaining; 1+, weak incomplete membranous staining, less than 10% of tumor cells; 2+, complete membranous staining, either uniform or weak in at least 10% of tumor cells; and 3+, uniform intense membranous staining in at least 30% of tumor cells. The case showing 2+ HER-2 IHC staining was evaluated by fluorescent in situ hybridization (FISH) to measure HER2 amplification. The result of E-cadherin was classified into total loss and partial loss. Total loss was defined as no immunostaining in tumor cells and partial loss was defined as incomplete weak membranous immunostaining. The IHC stain results of AR, p53, c-kit, CK5, CK5/6, vimentin, galectin-3 and EGFR were considered positive when more than 10% tumor cell were stained.
FISH analysis (Vysis pathvision c-erbB2 probe + DAKO FISH histology accessory kit) was performed manually. In brief, sections from formalin-fixed, paraffin-embedded tissue were mounted on Superfrost Plus slides, deparaffinized in xylene, and subsequently rehydrated in ethanol. Afterward, they were boiled for 10 min in pre-treatment solution, incubated with pepsin solution for 10 min, dehydrated in ethanol for 6 min, and finally air-dried. For hybridization, the buffered probe (HER2/neu and centromere 17) was brought onto the slide and protected by a coverslip that was sealed with rubber cement. For denaturation, slides were heated to 82℃ and incubated overnight at 45℃ in a dark humidified chamber. The rubber cement and coverslip were then removed, and the slides were transferred to stringent wash buffer for 10 min at 65℃. Afterward, they were dehydrated in ethanol for 6 min and air-dried. Finally, counterstaining was performed with 4',6-diamidino-2-phenylindole (DAPI). Counterstained slide was examined with an epifluorescence microscope (Olympus, Tokyo, Japan) equipped with a fluorescein, Cy3, DAPI filter set and 100 W mercury lamp. According to the Vysis manual (HER2 gene appears as orange and centromere 17 as green), the number of HER2 gene and centromere 17 were countered. We counted signals in at least 20 tumor nuclei in 2 separate regions of the tissue section. As proposed by the ASCO/CAP guideline,28 an absolute HER2 gene copy number lower than 4 or HER2 gene/chromosome 17 copy number ratio (HER2/Chr17 ratio) of less than 1.8 was considered HER2 negative; an absolute HER2 copy number between 4 and 6 or HER2/Chr17 ratio between 1.8 and 2.2 was considered HER2 equivocal; and an absolute HER2 copy number greater than 6 or HER2/Chr17 ratio higher than 2.2 was considered HER2 positive. Lymphocytes, fibroblasts, and normal ductal epithelial cells were used as internal controls.
Data were statistically processed using SPSS for Window version 12.0 (SPSS Inc., Chicago, IL, USA). For comparison of the groups, Student's t and Fisher's exact tests were used for continuous and categorical variables, respectively. Statistical significance was assumed when p < 0.05. Kaplan-Meier survival curves and log-rank statistics were employed to evaluate disease-free survival and overall survival.
Table 2 shows clinicopathologic characteristics of 117 cases of ILC. All patients were women with a mean age of 50.14 ± 9.07 years (range, 35-81 years). One hundred nine (93.2%) cases were classic type and 8 (6.8%) cases were pleomorphic type. Histologic grade was scored as follows: grade I, 46 (39.3%) cases, grade II, 68 (58.1%) cases; and grade III, 3 (2.6%) cases. The number of cases of nuclear grade 1 was 46 (39.3%), nuclear grade 2 was 63 (53.8%), and nuclear grade 3 was 8 (6.9%). Thirty six (30.8%) cases showed axillary lymph node metastasis. Tumor recurrence and distant metastasis were noted in 13 (11.1%) cases. When the study group was classified according to the IHC stain results of ER, PR and HER2, 8 (6.8%) cases were TNC. Clinicopathologic characteristics between triple negative and non-TNC are compared in Table 2. Histologic grade of TNC was higher than that of non-TNC (p = 0.019). There was a trend that nuclear grade of TNC was higher than that of non-TNC (p = 0.061). In histologic subtype, 1 (12.5%) case of pleomorphic type was TNC, and 7 (6.4%) cases of classic type were TNC, revealing that pleomorphic type showed higher proportion to be TNC than classic type although this difference did not reach statistical significance (p = 0.511). There was no statistical significance in age, lymph node metastasis, tumor recurrence, and distant metastasis between TNC and non-TNC.
We evaluated whether there was a prognostic difference between TNC and non-TNC group in ILC cases. In univariate analyses, there was no statistically significant difference in disease-free survival and overall survival (p = 0.982, and 0.424, respectively) (Fig. 1).
Table 3 demonstrates IHC characteristics of TNC (Fig. 2) and non-TNC (Fig. 3) in ILC. Galectin-3 was expressed with higher incidence in tumor cells of TNC (62.5%) than those of non-TNC (7.3%) (p = 0.000). There were no differences between nuclear and cytoplasmatic expression of galectin-3. However, there was a trend that cytoplasm expression was more predominant than nuclear expression. Galectin-3 was expressed with higher incidence in stromal cells of non-TNC (53.2%) than those of TNC (12.5%) (p = 0.029). AR was expressed with higher incidence in non-TNC (52.3%) than TNC (12.5%, p = 0.032). Vimentin, c-kit and EGFR which are known as markers of BLBC were expressed in 1 (12.5%) case of TNC. And CK5 and CK5/6, another marker of basal-like carcinoma, was not expressed in either TNC or non-TNC. p53 was expressed in only 6 (5.5%) cases of non-TNC. Total loss of E-cadherin was noted in 91 (83.5%) cases of non-TNC and 6 (75.0%) cases of TNC. And partial loss of E-cadherin was noted in 18 (16.5%) cases of non-TNC and 2 (25.0%) cases of TNC (p = 0.411).
Table 4 shows correlation analysis between galectin-3 expression in tumor cells and ER, PR, and HER2 expression. Out of 13 cases with galectin-3 expression, 5 (38.5%) cases were ER negative and 8 (61.5%) cases were ER positive. Out of 104 cases without galectin-3 expression, 4 (3.8%) cases were ER negative and 100 (96.2%) cases were ER positive. Therefore, higher incidence of ER negativity was noted in cases with galectin-3 expression (p = 0.001). In addition, higher incidence of PR negativity was noted in cases with galectin-3 expression (p = 0.039). There was no significant relationship between galectin-3 expression and HER-2 expression (p = 0.730).
Many researches have been ongoing since the concept of BLBC was raised through the gene expression profiling analysis of the breast cancer. Most of BLBC are reported to be ER, PR, and HER2 negative,12,14 rendering the entity to have the same clinical implication as TNC. However, the exact characteristics of TNC in ILC which comprise 5-15% of breast cancer are not so well-known because most of the previous intrinsic gene analysis were performed with at least 85% of ductal type carcinoma.11,19-21
In this study, 8 (6.8%) out of 117 cases of ILC were confirmed to be TNC through IHC and FISH. Generally, 15% of IDC are reported to be TNC,15 meaning that the proportion of TNC is lower in ILC than in IDC. This difference in the proportion of TNC in ILC and IDC can be explained by the fact that ER and PR are somehow more frequently expressed in ILC than in IDC.
This study also showed that the incidence of galectin-3 expression is higher in the tumor cells of TNC than in those of non-TNC (p = 0.000). In contrast, galectin-3 was expressed with higher incidence in the stromal cells of non-TNC than in those of TNC (p = 0.029). Galectin-3 is a 30 kD protein of non-integrin β-galactoside-binding lectin family29 and its role is associated with cell adhesion, migration, cell growth, cell cycle regulation, apoptosis, and cellular repair process.30-33 Although the role of galectin-3 in carcinogenesis is not exactly known, it is reported to be related with tumor progression and metastasis in head and neck,33 thyroid,34,35 gastric,30 and colon cancer.36 In breast cancer, galectin-3 showed down-regulation in IDC, but not in normal breast and benign disease.37 In addition, galectin-3 prevents apoptosis caused by nitric oxide in breast cancer cells.38 These results imply that galectin-3 is related with breast cancer development and progression. To our bestknowledge, galectin-3 expression in ILC, has not been well investigated until now. Moisa, et al.39 reported that the nuclear staining for galectin-3 is more frequent in ILC than in IDC, and galectin-3 expression in breast cancer stroma is related to an unfavorable prognosis. Our results show that galectin-3 is stained in both cytoplasm and nuclei, but more prominently in the cytoplasm, which is in concordance with the results reported by Moisa, et al. However, our study involved only ILC, rendering the comparison with IDC impossible. Moreover, even though the stromal expression of galectin-3 is reported to be related to the poor prognosis, it is derived from the study, including both IDC and ILC, and thus its comparison with our results is not plausible. In our study, 88.9% of ILC were galectin-3 negative, which is slightly higher than that in IDC (40-74%) shown in previous study.37 When ILC was classified into TNC and non-TNC, TNC showed significantly higher galectin-3 expression. In the present study, TNC showed higher incidence of high histologic grade (p = 0.016), and high nuclear grade (p = 0.061) than non-TNC, suggesting that TNC of ILC, as that of IDC, is a more aggressive histologic type. Paradoxically, however, galectin-3 was expressed more frequently in TNC. A previous study demonstrated that the expression of galectin-3 is increased in normal and benign duct, slightly decreased in low-grade of ductal carcinoma in situ (DCIS), and increased again in the tumor cells, mainly in periphery, as the tumor progressed to the high-grade comedo-DCIS and invasive carcinoma.40 This change of expression pattern of galectin-3 is suggested to cause increased invasive potential through interaction with the stromal tissue. The present study demonstrated that non-TNC of ILC showed galectin-3 negativity in the tumor cells and galectin-3 positivity in the stromal cells, whereas TNC of ILC showed galectin-3 positivity in the tumor cells and galectin-3 negativity in the stromal cells. Therefore, reciprocal expression of galectin-3 between the tumor cells and the stromal cells in TNC and non-TNC can be suggested in ILC. In addition, galectin-3 expression in the tumor cells was related to ER and PR negativity (p = 0.001, 0.039, respectively), therefore, the hormone receptor status may be involved in galectin-3 expression in ILC.
This study used IHC for vimentin, CK5, CK5/6, EGFR, and c-kit which are known as TNC marker of IDC,12,14,15 however, the marker which showed the expression confined only to TNC of ILC was EGFR, which was expressed in only 1 (16.7%) case of TNC showing low sensitivity. Vimentin was expressed in 1 case each of TNC (16.7%) and non-TNC (1.3%) and c-kit demonstrated similar incidence of expression in TNC (12.5%) and non-TNC (15.6%). Especially, CK5 and CK5/6 showed no expression in TNC and non-TNC. Previous studies which used CK5/6, but not CK5, reported that 5.4-28.6% of ILC showed basal CK expression.22-24 Although a consistent marker which can be used to define BLBC has not yet been established, most studies suggest that at least CK5 or CK5/6 expression is required to define BLBC.41,42 No expression of CK5/6 or CK5 in ILC can partly be explained by previous results, because the mean of IHC to identify BLBC has high specificity but low sensitivity.14 Therefore, CK5/6 or CK5 has a good positive predictive value, but not a good negative predictive value. The major limitation of this study is small number of TNC in ILC. ILC is a relatively rare breast cancer, compared to IDC, and the hormone receptor positive rate in ILC is higher than that of IDC. Therefore, the number of TNC in ILC should be smaller than that of TNC in IDC. The multi-institutional study on TNC in ILC should be performed to overcome this problem.
In conclusion, TNC in ILC showed some clinicopathologic and IHC characteristics, such as higher histologic grade, and increased expression of galectin-3, differing from non-TNC in ILC. TNC in ILC did not express CK5 and CK5/6, in contrast to TNC in IDC. Further multi-institutional study on TNC in ILC should be performed with large number of cases in order to understand the biology of these tumors.
Figures and Tables
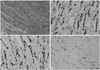
Fig. 1
Comparison of cumulative recurrence free survival (A) and cumulative overall survival (B) between TNC and non-TNC in ILC. TNC, triple negative carcinoma; ILC, invasive lobular carcinoma.

Fig. 2
(A) Triple negative phenotype of invasive lobular carcinoma shows scattered tumor cells with abundant eosinophilic cytoplasm and hyperchromatic enlarged nuclei (× 200, H&E). (B) The tumor cells represent galectin-3 expression in cytoplasm (× 200, galectin-3).

Fig. 3
(A) Non-triple negative phenotype of invasive lobular carcinoma shows linear strands of tumor cells in the fibrotic stroma (× 200, H&E). It expresses estrogen receptor (B) (× 200, ER) and progesterone receptor (C) (× 200, PR). (D) Peritumoral stromal cells demonstrate galectin-3 expresssion (× 200, galectin-3). ER, estrogen receptor; PR, progesterone receptor.
References
1. Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004. 6:R149–R156.

2. Yeatman TJ, Cantor AB, Smith TJ, Smith SK, Reintgen DS, Miller MS, et al. Tumor biology of infiltrating lobular carcinoma. Implications for management. Ann Surg. 1995. 222:549–559.
3. Rakha EA, El-Sayed ME, Powe DG, Green AR, Habashy H, Grainge MJ, et al. Invasive lobular carcinoma of the breast: response to hormonal therapy and outcomes. Eur J Cancer. 2008. 44:73–83.

4. Yoder BJ, Wilkinson EJ, Massoll NA. Molecular and morphologic distinctions between infiltrating ductal and lobular carcinoma of the breast. Breast J. 2007. 13:172–179.

5. Cleton-Jansen AM. E-cadherin and loss of heterozygosity at chromosome 16 in breast carcinogenesis: different genetic pathways in ductal and lobular breast cancer? Breast Cancer Res. 2002. 4:5–8.

6. Sarrió D, Pérez-Mies B, Hardisson D, Moreno-Bueno G, Suárez A, Cano A, et al. Cytoplasmic localization of p120ctn and E-cadherin loss characterize lobular breast carcinoma from preinvasive to metastatic lesions. Oncogene. 2004. 23:3272–3283.

7. du Toit RS, Locker AP, Ellis IO, Elston CW, Nicholson RI, Robertson JF, et al. An evaluation of differences in prognosis, recurrence patterns and receptor status between invasive lobular and other invasive carcinomas of the breast. Eur J Surg Oncol. 1991. 17:251–257.
8. Tubiana-Hulin M, Stevens D, Lasry S, Guinebretiére JM, Bouita L, Cohen-Solal C, et al. Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol. 2006. 17:1228–1233.

9. Lamovec J, Bracko M. Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol. 1991. 48:28–33.

10. Sastre-Garau X, Jouve M, Asselain B, Vincent-Salomon A, Beuzeboc P, Dorval T, et al. Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer. 1996. 77:113–120.

11. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001. 98:10869–10874.

12. Tischkowitz M, Brunet JS, Bégin LR, Huntsman DG, Cheang MC, Akslen LA, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007. 7:134.

13. Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006. 19:264–271.

14. Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004. 10:5367–5374.

15. Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007. 8:235–244.

16. Calza S, Hall P, Auer G, Bjöhle J, Klaar S, Kronenwett U, et al. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. Breast Cancer Res. 2006. 8:R34.

17. Jumppanen M, Gruvberger-Saal S, Kauraniemi P, Tanner M, Bendahl PO, Lundin M, et al. Basal-like phenotype is not associated with patient survival in estrogen-receptor-negative breast cancers. Breast Cancer Res. 2007. 9:R16.

18. Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005. 11:5678–5685.

19. Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003. 100:10393–10398.

20. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000. 406:747–752.

21. van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002. 415:530–536.
22. Abd El-Rehim DM, Pinder SE, Paish CE, Bell J, Blamey RW, Robertson JF, et al. Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol. 2004. 203:661–671.

23. Piekarski JH, Biernat W. Clinical significance of CK5/6 and PTEN protein expression in patients with bilateral breast carcinoma. Histopathology. 2006. 49:248–255.

24. Reis-Filho JS, Simpson PT, Martins A, Preto A, Gärtner F, Schmitt FC. Distribution of p63, cytokeratins 5/6 and cytokeratin 14 in 51 normal and 400 neoplastic human tissue samples using TARP-4 multi-tumor tissue microarray. Virchows Arch. 2003. 443:122–132.

25. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 2002. 41:154–161.

26. Cutler SJ, Black MM, Mork T, Harvei S, Freeman C. Further observations on prognostic factors in cancer of the female breast. Cancer. 1969. 24:653–667.

27. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999. 17:1474–1481.

28. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007. 25:118–145.

29. Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994. 269:20807–20810.

30. Bao Q, Hughes RC. Galectin-3 expression and effects on cyst enlargement and tubulogenesis in kidney epithelial MDCK cells cultured in three-dimensional matrices in vitro. J Cell Sci. 1995. 108:2791–2800.

31. Dagher SF, Wang JL, Patterson RJ. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci U S A. 1995. 92:1213–1217.

32. Le Marer N, Hughes RC. Effects of the carbohydrate-binding protein galectin-3 on the invasiveness of human breast carcinoma cells. J Cell Physiol. 1996. 168:51–58.

33. Sato S, Hughes RC. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J Biol Chem. 1992. 267:6983–6990.

34. Xu XC, el-Naggar AK, Lotan R. Differential expression of galectin-1 and galectin-3 in thyroid tumors. Potential diagnostic implications. Am J Pathol. 1995. 147:815–822.
35. Lotan R, Ito H, Yasui W, Yokozaki H, Lotan D, Tahara E. Expression of a 31-kDa lactoside-binding lectin in normal human gastric mucosa and in primary and metastatic gastric carcinomas. Int J Cancer. 1994. 56:474–480.

36. Schoeppner HL, Raz A, Ho SB, Bresalier RS. Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer. 1995. 75:2818–2826.

37. Castronovo V, Van Den Brûle FA, Jackers P, Clausse N, Liu FT, Gillet C, et al. Decreased expression of galectin-3 is associated with progression of human breast cancer. J Pathol. 1996. 179:43–48.

38. Moon BK, Lee YJ, Battle P, Jessup JM, Raz A, Kim HR. Galectin-3 protects human breast carcinoma cells against nitric oxide-induced apoptosis: implication of galectin-3 function during metastasis. Am J Pathol. 2001. 159:1055–1060.

39. Moisa A, Fritz P, Eck A, Wehner HD, Mürdter T, Simon W, et al. Growth/adhesion-regulatory tissue lectin galectin-3: stromal presence but not cytoplasmic/nuclear expression in tumor cells as a negative prognostic factor in breast cancer. Anticancer Res. 2007. 27:2131–2139.
40. Shekhar MP, Nangia-Makker P, Tait L, Miller F, Raz A. Alterations in galectin-3 expression and distribution correlate with breast cancer progression: functional analysis of galectin-3 in breast epithelial-endothelial interactions. Am J Pathol. 2004. 165:1931–1941.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download