Abstract
Hepatitis C virus (HCV), a non-cytopathic positive-stranded RNA virus, is one of the most common causes of chronic liver diseases such as chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. Upon HCV infection, the majority of patients fail to clear the virus and progress to chronic hepatitis C. Chemokines are small chemotactic cytokines that direct the recruitment of immune cells and coordinate immune responses upon viral infection. Chemokine production during acute HCV infection contributes to the recruitment of immune cells with antiviral effector functions and subsequent viral clearance. In chronic HCV infection, however, continuous production of chemokines due to persistent viral replication might result in incessant recruitment of inflammatory cells to the liver, giving rise to persistence of chronic inflammation and liver injury. In this review, we will summarize the roles of chemokines in acute and chronic settings of HCV infection and the clinical relevance of chemokines in the treatment of hepatitis C.
Hepatitis C virus (HCV) is a non-cytopathic positive-stranded RNA virus in the Flaviviridae family. It infects at least 170 million people worldwide and is a major cause of chronic liver disease.1-3 Upon HCV infection, 54 to 86% of patients progress to chronic hepatitis, and 20% of chronically infected patients develop long-term complications such as liver cirrhosis and hepatocellular carcinoma.4,5 On the other hand, 14 to 46% of HCV-infected patients undergo spontaneous viral clearance.4
The mechanisms leading to spontaneous viral clearance are not completely understood, while data support the role of T cell-mediated cellular immune responses as required for resolution of acute hepatitis C. Viral clearance after acute HCV infection is associated with the presence of robust, broad and persistent CD4+ and CD8+ T cell responses against HCV, whereas progression to chronic hepatitis C is associated with weak and narrow T cell responses to the virus.6-9 Given the essential role of effective cellular immune responses in the control of HCV, a better understanding of the factors that coordinate cellular immune responses could reveal novel therapeutic and preventive strategies.
Chemokines are small (8-12 kDa) chemotactic cytokines that direct the recruitment of immune cells to a specific site. Chemokines induce cell migration and activation by binding to specific G-protein coupled receptors containing seven transmembrane domains that are selectively expressed on different target cells, thereby orchestrating immune responses during acute and chronic inflammation.10,11 In addition to their essential roles in immune responses, chemokines also have diverse functions in angiogenesis, wound healing, hematopoiesis, lymphoid organ development, and regulation of embryonic development.11 Thus far, 50 different chemokines have been identified, and are subdivided into four families according to the number of amino acids between the N-terminal cysteine residues: the CC, the CXC, the CX3C, and the XC families. The chemokine receptors are also subdivided accordingly into four families: CCR, CXCR, CX3CR, and XCR.10-12
In order to clear viral infection, virus-specific T cells must traffic to the virus-infected tissues to recognize virus-infected cells and to exert effector functions. The migration of lymphocytes from blood into the peripheral inflammatory tissues is a complex multistep process involving adhesion, rolling, triggering, and transendothelial migration (diapedesis). Chemokines and their receptors are the key players in this whole process.13,14
Chemokines have been shown to play an important role in the pathogenesis of HCV infection, as reflected in their elevated levels in the peripheral blood and the liver. During HCV infection, T helper 1 (Th1) inflammatory CD4+ T cells and CD8+ T cells predominate in the liver, and liver-infiltrating T cells express Th1-associated chemokine receptors such as CXCR3 and CCR5.15-21 In addition to recruiting subsets of lymphocytes, there is evidence for compartmentalization of the recruited lymphocytes within the liver as well. For example, CCR5 seems to recruit lymphocytes to portal tracts, and CXCR3 appears to be essential for the recruitment of lymphocytes into the parenchyma.22-25 Hence, Th1-associated chemokines might be of particular importance in the immunopathogenesis of acute and chronic HCV infection.
CXCR3 is a receptor for CXCL9 (monokine induced by interferon-γ, MIG), CXCL10 (interferon-γ-inducible protein-10, IP-10) and CXCL11 (interferon-inducible T-cell α chemoattractant, I-TAC) (Fig. 1A), and is known to be expressed in Th1 CD4+ T cells and CD8+ T cells, especially in effector and effector memory subsets. CCR5 is a receptor for CCL3 (macrophage inflammatory protein-1α, MIP-1α), CCL4 (macrophage inflammatory protein-1β, MIP-1β) and CCL5 (regulated on activation normal T cell expressed and secreted, RANTES) though CCL3 and CCL5 bind to other receptors redundantly (Fig. 1B). CCR5 is also expressed in effector and effector memory subsets of Th1 CD4+ T cells and CD8+ T cells.
Given the importance of Th1 CD4+ T cells and CD8+ T cells in HCV infection, we will focus mainly on Th1-associated chemokines in acute and chronic settings of HCV infection, and the clinical relevance of chemokines in treating hepatitis C.
Acute hepatitis C is defined as the first 6 months after infection with HCV. However, symptomatic acute hepatitis C occurs in only about 15% of patients, and anti-HCV antibody is not detected at the onset of symptoms in about a third of infected patients.26 Accordingly, relatively a little information is available on the role of chemokines in acute HCV infection, and much of our understanding of the regulation of chemokines in acute HCV infection comes from studies in HCV-infected chimpanzee models,27-29 or in the setting of seroconversion in high-risk individuals such as injection drug users (IDUs).30
In the early phase of HCV infection, pattern recognition receptors trigger production of type I interferon (IFN) in response to HCV RNA,24,27,31-34 which is followed by production of Th1-associated chemokines two to eight weeks after HCV infection.27,33 It is anticipated that this early production of chemokines immediately induces liver infiltration of HCV-specific CD8+ T cells that target and lyse the virus-infected hepatocytes and cause liver injury. In fact, however, liver infiltration of T cells and liver injury are not observed before 8-12 weeks after HCV infection, when chemokines are already being produced. A recent study showed that the delayed recruitment of CD8+ T cells is due to late priming of HCV-specific CD8+ T cells at 8-12 weeks, not to impaired production of chemokines. Fig. 2A illustrates the production of CXCR3- and CCR5-associated chemokines, the recruitment of CXCR3+/CCR5+ HCV-specific T cells, and their contribution to viral clearance and liver injury.
Several studies have assessed the dynamic changes in the expression of chemokines throughout the course of acute HCV infection in humans and chimpanzees.27-30,33,35 The chimpanzee studies report increased expression of CXCR3- and CCR5-associated chemokines in the liver and the plasma.27-29 In a study of naïve chimpanzees following HCV infection, intrahepatic mRNA levels of CCL3 were upregulated in the animals that spontaneously eradicated the virus compared to those that developed chronic infection.29 In another study of chimpanzees rechallenged with different genotypes of HCV after spontaneous clearance of initial HCV infection, intrahepatic mRNA levels of CXCL9 and CXCL10 were shown to be significantly elevated.28 Early elevation of intrahepatic mRNA levels and plasma levels of CXCL10, CXCL11, CCL4, and CCL5 were also reported in a recent study of chimpanzees with acute HCV infection.27 This increase began and was maintained 2-8 weeks after HCV infection, earlier than liver infiltration of T cells and serum ALT elevation. In addition to CXCR3- and CCR5-associated chemokines, CXCL8 (IL-8) expression is also known to be induced by in vitro HCV infection.36
In a recent study of IDUs with acute HCV infection, plasma levels of CXCL9, CXCL10, and CXCL11 were elevated, whereas CCL3 and CCL4 levels did not change significantly during acute HCV infection.30 A comparison of self-limited and chronically evolved subjects did not show any difference in the onset and the kinetics of early chemokine production,30,33 suggesting that chemokine production most likely does not predict the outcome of acute HCV infection.
Despite its actions in antiviral immune responses, chemokine production may be impeded by HCV per se. For instance, HCV E2 protein attenuates the secretion of CCL3 and CCL4,37 and HCV NS3/4A protein complex downregulates the production of CXCL10, CCL5, and CXCL8.38
To summarize, during the acute phase of HCV infection, Th1-associated chemokines are produced and HCV-specific T cells are recruited to the liver for viral clearance. Although chemokine production does not seem to predict the outcome of acute HCV infection, evasion mechanisms utilized by HCV interfere with the production of chemokines.
Chronic hepatitis C is defined as infection with HCV persisting for more than 6 months, and is characterized by infiltration of inflammatory cells.18
Although chemokine production during acute HCV infection may contribute to the recruitment of antiviral T cells and to the subsequent viral elimination, chronic HCV infection is a completely different story. Chemokines have an essential role in the development of inflammation in chronic HCV infection as reflected in their elevated levels in the peripheral blood and the liver.15-21 Constantly increased production of chemokines caused by persistent HCV RNA replication results in continuous recruitment of inflammatory cells, both virus-specific and non-specific, to the liver and subsequent chronic liver injury without clearing the virus (Fig. 2B). Many of the liver-infiltrating HCV-specific T cells isolated from chronic HCV patients have been shown to be functionally exhausted, yet they may play a part in the persistence of chronic inflammation that leads to continuous liver injury and long-term complications.
Several chemokines have been correlated with hepatic inflammation in chronic HCV infection. Among the CXC chemokines, CXCL10 expression is increased in the liver and the peripheral blood.19,24,39-43 Intrahepatic mRNA levels of CXCL10 were correlated with lobular inflammation,24,44 and plasma levels of CXCL10 were correlated with necroinflammation.42,43 It is produced by hepatocytes and liver sinusoidal endothelial cells (LSECs).19,41 CXCL9 is also increased in the liver and the peripheral blood.19,39,40,45 It is produced by LSECs and its levels are correlated with the grade of inflammation.19,39,40,45 CXCL11 is produced mainly by hepatocytes.32 Intrahepatic mRNA levels of CXCL11 are significantly increased, and they were correlated with both portal and lobular inflammation.32
Among the CC chemokines, CCR5-associated chemokines are strongly expressed on portal endothelium. CCL5, produced by hepatocytes, LSECs and biliary epithelial cells, is increased in the liver and was correlated with the grade of inflammation.45,46 CCL3 and CCL4 are also increased in the liver and the peripheral blood, and are produced by hepatocytes, LSECs and biliary epithelial cells.24,39 As a result, CXCR3+/CCR5+ T cells, including non-specific cells, are recruited to the hepatic lobule and portal tracts, where they exert effector functions such as cytotoxicity and cytokine/chemokine production and induce chronic liver injury.
This phenomenon, by which chemokine production during the acute and chronic infection has different consequences, has been studied in other small animal models such as murine infection of the central nervous system (CNS) with mouse hepatitis virus (MHV).47 In this model, intracerebral MHV infection causes acute encephalomyelitis characterized by robust cell-mediated immune responses in which both T cells and macrophages infiltrate the CNS and exert antiviral functions, followed by a chronic demyelinating disease characterized by continuous recruitment of leukocytes and their accumulation within the CNS. Chemokines play diverse roles in this process. During the acute phase, CXCL10 and CXCL9 recruit effector T cells to the CNS, bringing about antiviral functions. During the chronic phase, on the contrary, CXCL10 and CCL5 recruit inflammatory cells to the sites of viral persistence, leading to myelin destruction.
Along with CXCR3- and CCR5-associated chemokines, several other chemokines also have been correlated with hepatic inflammation in chronic HCV infection. CXCL16 expression is increased in the liver, and its receptor, CXCR6, is expressed on a large fraction of activated liver-infiltrating inflammatory cells such as CD4+ T cells, CD8+ T cells, NK cells, and NKT cells.15 CXCL12, a CXCR4-associated chemokine, is also elevated in the liver and the peripheral blood.48 CXCL12 is produced by the LSECs and the biliary epithelial cells of the inflamed liver, and its expression was correlated with disease severity and fibrosis in chronic HCV infection.48 Notably, CCL21, a ligand of CCR7, is upregulated in the HCV-infected liver,49 suggesting its involvement in the organization of inflammatory lymphoid follicles in the liver that are frequently observed in chronic hepatitis C.
To summarize, Th1-associated chemokines might be required for mounting effective antiviral immune responses in acute HCV infection. During chronic HCV infection, however, Th1-associated chemokines might contribute to the recruitment of inflammatory cells, which are not necessarily virus-specific, to the liver. The recruited inflammatory cells cause chronic liver injury rather than viral clearance. Hence, the involvement of chemokines needs to be considered differently during the acute and chronic stages of HCV infection (Fig. 2).
Several polymorphisms in chemokine and chemokine receptor genes have been associated with different outcomes of HCV infection. CCR5Δ32, a polymorphism defined by homozygosity for a 32-bp deletion in the CCR5 gene, has shown conflicting associations for control of HCV. Historically, CCR5 was found to be an essential co-receptor of HIV binding, and thus individuals homozygous for CCR5Δ32 were protected from HIV infection. Even though CCR5 is not a co-receptor of HCV, CCR5 polymorphism was regarded as a predictor for the outcome of HCV infection and the course of HCV-related liver disease. A study by Woitas, et al.50 found an increased prevalence of chronic HCV infection among patients with CCR5Δ32 polymorphism compared with healthy controls. By contrast, Mangia, et al.51 and Zhang, et al.52 did not find any association between CCR5Δ32 and HCV infection. Furthermore, Goulding, et al.53 reported that the presence of CCR5Δ32 was significantly associated with spontaneous viral clearance. In addition, in a study by Hellier, et al.54, CCR5Δ32 was not associated with spontaneous viral clearance, but a polymorphism at position 2132 of CCR5 promoter was weakly associated with susceptibility to chronic HCV infection. This study found that chronic HCV infection was not associated with polymorphisms within chemokines such as CCL3, CCL5, and CCL2 (monocyte chemotactic protein-1, MCP-1).54
On the other hand, a polymorphism of CXCL11, defined by a 5-bp deletion in CXCL11 promoter, was found to be associated with chronic HCV infection.55 This polymorphism significantly reduced the transcriptional activity of the CXCL11 promoter, thereby impairing T cell migration and causing failure to clear the virus.
As the importance of chemokines in acute HCV infection has been emphasized earlier, it may be questioned whether chemokine levels during acute HCV infection can predict spontaneous viral clearance or progression to chronic hepatitis C. However, recent studies in chimpanzees and IDUs have shown that chemokine levels were increased in the acute phase regardless of the outcome of acute HCV infection.27,30
Nevertheless, chemokines are considered to be useful biomarkers of treatment outcomes of hepatitis C. Several studies have found that elevated plasma levels of pre-treatment CXCL10 are correlated with therapeutic non-response in chronic hepatitis C patients treated with a combination of pegylated-IFN and ribavirin.40,43,56-58 This observation seems to be paradoxical as CXCL10 is a Th1-associated chemokine and recruits Th1 CD4+ and CD8+ T cells. A recent study by Casrouge, et al.59 proposed an interesting explanation to this paradoxical phenomenon. Increased CXCL10 in non-responder patients is in fact the N-terminal-cleaved-form of CXCL10 that acts as a CXCR3 antagonist. Thus, the N-terminal-cleaved-form of CXCL10 inhibits migration of effector T cells from the blood into the liver. Further validation is needed, however, in order to use pre-treatment plasma levels of CXCL10 as a predictor of treatment outcomes of hepatitis C.
Several other chemokines have also been suggested as candidates for biomarkers of treatment outcome. Elevated pre-treatment plasma levels of CCL11 (eotaxin) and CCL4 were associated with sustained virological response,60,61 whereas increased plasma levels of CXCL8 were associated with resistance to the standard antiviral treatment.62
Chemokines are important in lymphocyte recruitment to the liver in HCV infection. They play an essential part in orchestrating the antiviral immune responses during acute HCV infection. CXCR3-associated chemokines (such as CXCL9, CXCL10, and CXCL11) and CCR5-associated chemokines (such as CCL3, CCL4, and CCL5) seem to be required for effective antiviral T cell responses during the acute phase of HCV infection. In chronic HCV infection, on the contrary, HCV replicates persistently within the hepatocytes, thus sustaining chemokine production. As a result, continuous recruitment of inflammatory cells to the liver is provoked, and liver injury continues without viral clearance. Many of the liver-infiltrating HCV-specific lymphocytes isolated from chronic HCV patients have been shown to be functionally exhausted, yet they may contribute to the persistence of chronic inflammation and liver injury.
In addition to their roles in liver inflammation, chemokines may also be used as biomarkers of antiviral treatment outcomes of hepatitis C. An antagonist form of CXCL10 was recently identified as a negative predictive marker to the current standard antiviral treatment. Other chemokines have also been suggested as candidates for biomarkers of treatment outcomes. CCL11 and CCL4 were suggested as positive predictive markers, while CXCL8 was suggested as a negative predictive marker.
Several polymorphisms in chemokine and chemokine receptor genes have been associated with different outcomes of HCV infections. CCR5Δ32 has shown conflicting associations with the outcomes of HCV infection, whereas polymorphisms within chemokines such as CCL3, CCL5, and CCL2 were not associated with chronic HCV infection. Meanwhile, a polymorphism of CXCL11, defined by a 5-bp deletion in CXCL11 promoter, was associated with chronic HCV infection.
Thus, further studies of chemokines in HCV infection would contribute not only to understanding of immunopathogenesis of hepatitis C but also to the development of novel therapeutics and predictive markers.
Figures and Tables
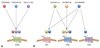 | Fig. 1Schematic overview of Th1-associated chemokines and their receptors. Representative Th1-associated chemokine receptors are CXCR3 and CCR5. (A) CXCR3 is a receptor for CXCL9, CXCL10 and CXCL11, and is known to be expressed in Th1 CD4+ T cells and CD8+ T cells, especially in effector and effector memory subsets. (B) CCR5 is a receptor for CCL3, CCL4 and CCL5, though CCL3 and CCL5 bind to other receptors redundantly. CCR5 is also expressed in effector and effector memory subsets of Th1 CD4+ T cells and CD8+ T cells. MIG, monokine induced by interferon-γ; IP-10, interferon-γ-inducible protein-10; I-TAC, interferon-inducible T-cell α chemoattractant; MIP-1α, macrophage inflammatory protein-1α; MIP-1β, macrophage inflammatory protein-1β; RANTES, regulated on activation normal T cell expressed and secreted; Th1, T helper 1. |
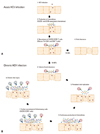 | Fig. 2Roles of chemokines in acute and chronic HCV infection. (A) During acute HCV infection, CXCR3- and CCR5-associated chemokines are produced at 2-8 weeks after HCV infection. The released chemokines contribute to viral clearance by recruiting effector Th1 CD4+ and CD8+ T cells. (B) In the majority of cases, however, viral clearance fails to accomplish, and HCV replication persists within the liver. The constant increase in the production of chemokines due to persistent HCV RNA replication results in continuous recruitment of inflammatory cells, both virus-specific and non-specific, to the liver and subsequent chronic liver injury without clearing the virus. HCV, hepatitis C virus. |
ACKNOWLEDGEMENTS
This work was supported by National Research Foundation grants funded by the Korea government (Ministry of Education, Science and Technology) (2010-0004539 and 2010-0030075).
References
1. Hepatitis C: global prevalence. Wkly Epidemiol Rec. 1997. 72:341–344.
2. Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005. 5:558–567.

3. Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000. 20:1–16.

4. Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002. 36:S35–S46.
6. Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000. 191:1499–1512.

7. Grüner NH, Gerlach TJ, Jung MC, Diepolder HM, Schirren CA, Schraut WW, et al. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis. 2000. 181:1528–1536.

8. Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY, et al. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999. 10:439–449.

9. Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, et al. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A. 2002. 99:15661–15668.

10. Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000. 95:3032–3043.

11. Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000. 18:217–242.

12. Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000. 12:121–127.
14. Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994. 76:301–314.

15. Boisvert J, Kunkel EJ, Campbell JJ, Keeffe EB, Butcher EC, Greenberg HB. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J Hepatol. 2003. 38:67–75.

16. Heydtmann M, Hardie D, Shields PL, Faint J, Buckley CD, Campbell JJ, et al. Detailed analysis of intrahepatic CD8 T cells in the normal and hepatitis C-infected liver reveals differences in specific populations of memory cells with distinct homing phenotypes. J Immunol. 2006. 177:729–738.

17. Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, et al. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol. 2002. 160:347–355.

18. Leroy V, Vigan I, Mosnier JF, Dufeu-Duchesne T, Pernollet M, Zarski JP, et al. Phenotypic and functional characterization of intrahepatic T lymphocytes during chronic hepatitis C. Hepatology. 2003. 38:829–841.

19. Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999. 163:6236–6243.
20. Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998. 391:344–345.

21. Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998. 101:746–754.

22. Ajuebor MN, Hogaboam CM, Le T, Proudfoot AE, Swain MG. CCL3/MIP-1alpha is pro-inflammatory in murine T cell-mediated hepatitis by recruiting CCR1-expressing CD4(+) T cells to the liver. Eur J Immunol. 2004. 34:2907–2918.

23. Curbishley SM, Eksteen B, Gladue RP, Lalor P, Adams DH. CXCR 3 activation promotes lymphocyte transendothelial migration across human hepatic endothelium under fluid flow. Am J Pathol. 2005. 167:887–899.

24. Harvey CE, Post JJ, Palladinetti P, Freeman AJ, Ffrench RA, Kumar RK, et al. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003. 74:360–369.

25. Murai M, Yoneyama H, Harada A, Yi Z, Vestergaard C, Guo B, et al. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999. 104:49–57.

27. Shin EC, Park SH, Demino M, Nascimbeni M, Mihalik K, Major M, et al. Delayed Induction, Not Impaired Recruitment of Specific CD8(+) T Cells, Causes the Late Onset of Acute Hepatitis C. Gastroenterology. 2011. 141:686–695.

28. Lanford RE, Guerra B, Chavez D, Bigger C, Brasky KM, Wang XH, et al. Cross-genotype immunity to hepatitis C virus. J Virol. 2004. 78:1575–1581.

29. Major ME, Dahari H, Mihalik K, Puig M, Rice CM, Neumann AU, et al. Hepatitis C virus kinetics and host responses associated with disease and outcome of infection in chimpanzees. Hepatology. 2004. 39:1709–1720.

30. Zeremski M, Hooker G, Shu MA, Winkelstein E, Brown Q, Des Jarlais DC, et al. Induction of CXCR3- and CCR5-associated chemokines during acute hepatitis C virus infection. J Hepatol. 2011.

31. Decalf J, Fernandes S, Longman R, Ahloulay M, Audat F, Lefrerre F, et al. Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients. J Exp Med. 2007. 204:2423–2437.

32. Helbig KJ, Ruszkiewicz A, Semendric L, Harley HA, McColl SR, Beard MR. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology. 2004. 39:1220–1229.

33. Shin EC, Seifert U, Kato T, Rice CM, Feinstone SM, Kloetzel PM, et al. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J Clin Invest. 2006. 116:3006–3014.

34. Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M, et al. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci U S A. 2010. 107:7431–7436.

35. Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009. 83:3719–3733.

36. Wagoner J, Austin M, Green J, Imaizumi T, Casola A, Brasier A, et al. Regulation of CXCL-8 (interleukin-8) induction by double-stranded RNA signaling pathways during hepatitis C virus infection. J Virol. 2007. 81:309–318.

37. Volkov Y, Long A, Freeley M, Golden-Mason L, O'Farrelly C, Murphy A, et al. The hepatitis C envelope 2 protein inhibits LFA-1-transduced protein kinase C signaling for T-lymphocyte migration. Gastroenterology. 2006. 130:482–492.

38. Sillanpää M, Kaukinen P, Melén K, Julkunen I. Hepatitis C virus proteins interfere with the activation of chemokine gene promoters and downregulate chemokine gene expression. J Gen Virol. 2008. 89:432–443.

39. Apolinario A, Diago M, Lo Iacono O, Lorente R, Pérez C, Majano PL, et al. Increased circulating and intrahepatic T-cell-specific chemokines in chronic hepatitis C: relationship with the type of virological response to peginterferon plus ribavirin combination therapy. Aliment Pharmacol Ther. 2004. 19:551–562.

40. Butera D, Marukian S, Iwamaye AE, Hembrador E, Chambers TJ, Di Bisceglie AM, et al. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood. 2005. 106:1175–1182.

41. Narumi S, Tominaga Y, Tamaru M, Shimai S, Okumura H, Nishioji K, et al. Expression of IFN-inducible protein-10 in chronic hepatitis. J Immunol. 1997. 158:5536–5544.
42. Nishioji K, Okanoue T, Itoh Y, Narumi S, Sakamoto M, Nakamura H, et al. Increase of chemokine interferon-inducible protein-10 (IP-10) in the serum of patients with autoimmune liver diseases and increase of its mRNA expression in hepatocytes. Clin Exp Immunol. 2001. 123:271–279.

43. Romero AI, Lagging M, Westin J, Dhillon AP, Dustin LB, Pawlotsky JM, et al. Interferon (IFN)-gamma-inducible protein-10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J Infect Dis. 2006. 194:895–903.

44. Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008. 48:1440–1450.

45. Apolinario A, Majano PL, Alvarez-Pérez E, Saez A, Lozano C, Vargas J, et al. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002. 97:2861–2870.

46. Kusano F, Tanaka Y, Marumo F, Sato C. Expression of C-C chemokines is associated with portal and periportal inflammation in the liver of patients with chronic hepatitis C. Lab Invest. 2000. 80:415–422.

47. Glass WG, Chen BP, Liu MT, Lane TE. Mouse hepatitis virus infection of the central nervous system: chemokine-mediated regulation of host defense and disease. Viral Immunol. 2002. 15:261–272.

48. Wald O, Pappo O, Safadi R, Dagan-Berger M, Beider K, Wald H, et al. Involvement of the CXCL12/CXCR4 pathway in the advanced liver disease that is associated with hepatitis C virus or hepatitis B virus. Eur J Immunol. 2004. 34:1164–1174.

49. Bonacchi A, Petrai I, Defranco RM, Lazzeri E, Annunziato F, Efsen E, et al. The chemokine CCL21 modulates lymphocyte recruitment and fibrosis in chronic hepatitis C. Gastroenterology. 2003. 125:1060–1076.

50. Woitas RP, Ahlenstiel G, Iwan A, Rockstroh JK, Brackmann HH, Kupfer B, et al. Frequency of the HIV-protective CC chemokine receptor 5-Delta32/Delta32 genotype is increased in hepatitis C. Gastroenterology. 2002. 122:1721–1728.

51. Mangia A, Santoro R, D'agruma L, Andriulli A. HCV chronic infection and CCR5-delta32/delta32. Gastroenterology. 2003. 124:868–869.
52. Zhang M, Goedert JJ, O'Brien TR. High frequency of CCR5-delta32 homozygosity in HCV-infected, HIV-1-uninfected hemophiliacs results from resistance to HIV-1. Gastroenterology. 2003. 124:867–868.

53. Goulding C, McManus R, Murphy A, MacDonald G, Barrett S, Crowe J, et al. The CCR5-delta32 mutation: impact on disease outcome in individuals with hepatitis C infection from a single source. Gut. 2005. 54:1157–1161.

54. Hellier S, Frodsham AJ, Hennig BJ, Klenerman P, Knapp S, Ramaley P, et al. Association of genetic variants of the chemokine receptor CCR5 and its ligands, RANTES and MCP-2, with outcome of HCV infection. Hepatology. 2003. 38:1468–1476.

55. Helbig KJ, George J, Beard MR. A novel I-TAC promoter polymorphic variant is functional in the presence of replicating HCV in vitro. J Clin Virol. 2005. 32:137–143.

56. Lagging M, Romero AI, Westin J, Norkrans G, Dhillon AP, Pawlotsky JM, et al. IP-10 predicts viral response and therapeutic outcome in difficult-to-treat patients with HCV genotype 1 infection. Hepatology. 2006. 44:1617–1625.

57. Diago M, Castellano G, García-Samaniego J, Pérez C, Fernández I, Romero M, et al. Association of pretreatment serum interferon gamma inducible protein 10 levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1 infected patients with chronic hepatitis C. Gut. 2006. 55:374–379.

58. Askarieh G, Alsiö A, Pugnale P, Negro F, Ferrari C, Neumann AU, et al. Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C virus RNA and overall viral response to therapy in chronic hepatitis C. Hepatology. 2010. 51:1523–1530.

59. Casrouge A, Decalf J, Ahloulay M, Lababidi C, Mansour H, Vallet-Pichard A, et al. Evidence for an antagonist form of the chemokine CXCL10 in patients chronically infected with HCV. J Clin Invest. 2011. 121:308–317.

60. Vargas A, Berenguer J, Catalán P, Miralles P, López JC, Cosín J, et al. Association between plasma levels of eotaxin (CCL-11) and treatment response to interferon-alpha and ribavirin in HIV/HCV co-infected patients. J Antimicrob Chemother. 2010. 65:303–306.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download