Abstract
Purpose
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) scans are frequently performed for the screening or staging of malignant tumors. This study aimed to assess the usefulness of 18F-FDG PET/CT in detection of gastric cancer recurrence after curative gastrectomy.
Materials and Methods
Eighty nine patients who had undergone curative gastrectomy due to gastric cancer and had 18F-FDG PET/CT and contrast CT scans within 2 weeks for surveillance in asymptomatic patients (n = 11) or to clarify suspected recurrence (n = 78) were consecutively collected and retrospectively analyzed. They had clinical follow-up for at least 12 months after PET/CT and CT scans.
Results
Fifteen of the 89 patients (16.9%) were diagnosed with recurrent gastric cancer in 21 organs. Forty one organs showed an increase in FDG uptake, and only 9 of these organs were diagnosed with recurrent gastric cancer by 18F-FDG PET/CT. The sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of the 18F-FDG PET/CT were 42.9%, 59.7%, 29.3%, 78.2%, and 57.3%, respectively. On the CT scan, 18 of 21 recurrent gastric cancers were detected, and 7 cases were in agreement with the 18F-FDG PET/CT. The sensitivity and specificity of the CT scan were 85.8% and 87.3%, respectively, which are superior to the 18F-FDG PET/CT. When we diagnosed a recurrence based on either 18F-FDG PET/CT or CT scans, the sensitivity increased to 95.2% and the specificity decreased to 45.6%, when compared with the contrast CT scan alone.
Radical surgical resection of gastric cancer is a considered curative treatment. However, the reported recurrence rate after curative surgery ranges between 22% and 60%.1,2 Therefore, early detection of recurrence is important in an effort to improve prognosis.
To detect recurrences, endoscopy, other imaging modalities, and tumor markers have been used. However, endoscopic diagnosis has a high sensitivity in detecting intra-luminal recurrences of gastric cancer, and other imaging studies, such as a computed tomography (CT) scan or 18f-fluorodeoxyglucose positron emission (18F-FDG PET) scan, are used in diagnosing local recurrences or distant metastases. A CT scan is the most common method for detecting gastric cancer recurrences, and has 76% to 93% sensitivity for T staging,3 and 52% to 73% sensitivity for N staging, but is of limited diagnostic value in diagnosing peritoneal or hematogenous metastases.4-6
A PET scan is another imaging modality that reflects cancer cell metabolism via glucose utilization using 18f-fluorodeoxyglucose (18F-FDG) as a tracer. 18F-FDG PET scan combined with CT scan (18F-FDG PET/CT) can yield more accurate information by stereographic reconstruction. 18F-FDG PET/CT scans are frequently performed for staging of lung, colorectal, breast, head and neck carcinomas, and lymphomas. In gastric cancer, the FDG uptake of cancer cells is relatively low and the reproducibility is limited, more than in other malignancies. Indeed, there are only a few reports of the clinical role of 18F-FDG PET/CT scans in the detection of gastric cancer recurrence.7-11 However, the role of 18F-FDG PET/CT scans in detecting gastric cancer recurrence after curative gastrectomy is unclear. Therefore, we assessed the utility of 18F-FDG PET/CT scans in the evaluation of gastric cancer recurrence after curative gastrectomy.
The cases of 89 patients, who had undergone curative gastrectomy for gastric cancer and had 18F-FDG PET/CT followed by abdominopelvic contrast CT scans or vice versa within 2 weeks of the operation between January 2006 and September 2007, were consecutively recruited. They had 18F-FDG PET/CT for surveillance in asymptomatic patients or to clarify suspected recurrence. Any patient who had clinical follow-up for less than 12 months after the PET/CT and CT scans was excluded. This study was designed to be a retrospective analysis based on medical records and was approved by the institutional review board. The 6th UICC TNM classification was used for gastric staging.12
The patients fasted at least 4 h prior to intravenous injection of 370-666 MBq [10-18 mCi (0.14 mCi/kg)] 18F-FDG. Blood glucose levels were checked in patients with diabetes and patients who did not know their blood glucose levels prior to the injection of 18F-FDG. A PET/CT scan was performed only when blood glucose levels did not exceed 150 mg/dL (8.3 mmol/L). Data acquisition was done by an integrated PET/CT system (Philips Gemini, DA Best, the Netherlands) 1 h after the 18F-FDG injections. CT scanning was performed prior to the PET scan from the head to the pelvic floor with 120 kVp, 250 mA, and a 5.3 mm section thickness. Next, the PET scan was performed with a 5-min emission acquisition per imaging level and the images were reconstructed. PET image data was acquired by imaging reconstruction using a Row Action Maximum Likelihood Algorithm (RAMLA). A board certified nuclear radiologist reviewed the 18F-FDG PET/CT scans. Strong and focal FDG uptake combined with a delayed image was indicative of a recurring malignant lesion, but diffuse or segmental patterns without focally increased accumulation were interpreted as physiologic uptakes.
The patients fasted at least 6 h prior to the CT scan, and ingested 600-800 mL of oral contrast. Scanning from above the diaphragm to the greater trochanter was performed using a 16-row multi-slice CT unit (Sensation 16; Siemens Medical Solutions, Erlangen, Germany), with 120 kVp, 300 mA, and 5 mm section thickness at 7 mm/sec table speed.
Eighty nine consecutive patients who had undergone curative gastrectomy for gastric cancer and had 18F-FDG PET/CT and CT scans between January 2006 and September 2007 as a follow-up study were enrolled. Sixty two patients were male and 27 patients were female. Patient age ranged from 27-82 years, the mean age being 56.4 years old (± 12.5). The duration of follow-up after surgery ranged from 12-125 months, and the mean duration was 34.5 months (± 24.9). The post-surgery time to 18F-FDG PET/CT ranged from 3-110 months, and the mean interval was 24.7 months (± 22.7). Seventy eight patients had 18F-FDG PET/CT for clarification of a suspected recurrence while 11 patients had 18F-FDG PET/CT for surveillance without suspicion of recurrence.
The post-operative pathologic stages of gastric cancer were as follows: Ia, 9 (10.1%); Ib, 10 (11.2%); II, 19 (21.3%); IIIa, 28 (31.5%); IIIb, 10 (11.2%); and IV, 13 (14.6%). Patients with stages Ia and Ib, who had 18F-FDG PET/CT were suspicious for recurrence based on elevated tumor marker levels or regional LN enlargement on the contrast CT scan. All 10 patients with stage IIIb showed T3N2M0. Patients with stage IV included 5 patients with T4NxM0 and 8 patients with TxN3M0. All patients with stage III or IV had no evidence of unresectable lymph nodes or distant metastasis before operation. Adenocarcinoma was the most common histologic type (n = 65; 73.0%), followed by signet ring cell type (n = 17; 19.1%), and mucinous cell type (n = 7; 7.9%) (Table 1).
Patients whom recurrence was not suspected were staged as 5 Ia, 3 Ib, and 3 IIIa.
Recurrences were diagnosed in 15 of 89 patients (16.9%), all in the suspected group. The pathologic stages were as follows: II, 2 (13.3%); IIIa, 7 (46.7%); IIIb, 2 (13.3%); and IV, 4 (26.7%). Histologically, nine patients (60.0%) showed poorly differentiated adenocarcinoma and five patients (33.3%) showed signet ring cell type, and one patient (6.7%) showed moderately differentiated adenocarcinoma (Table 1).
The 15 patients' recurrences involved 21 different anatomic sites. Among these, 3 involved the regional bowel, 4 involved the peritoneum, 2 involved the liver, 3 involved the regional lymph node, 4 involved the distant lymph node, and 5 involved other organs, including the pancreas, bile duct, ovaries, ureters, and brain (Table 2).
Forty one sites showed increased FDG uptake. There were nine true recurring sites, and 29 false positive sites; three other sites were one primary thyroid cancer and two primary lung cancers.
A prominently high false positive rate (22 of 22 sites; 100%) occurred in the bowel, including the remnant stomach, small intestine, and large intestine. In the lymph nodes, 7 out of 14 sites (50.0%) were diagnosed as true recurrences. In the liver, two of two suspicious sites were diagnosed as true recurrences (Table 3).
In addition, two lymph nodal recurrences were only detected by 18F-FDG PET/CT. There was one case involving cervical lymph nodal metastasis without locoregional recurrence that was detected by 18F-FDG PET/CT (Fig. 1).
According to histologic type, 70% (7 of 10 sites) of adenocarcinomas had increased FDG uptake. However, none of the recurrent signet ring cell carcinomas had FDG uptake.
The sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of 18F-FDG PET/CT scans for detecting gastric cancer recurrence after curative gastrectomy were 42.9%, 59.7%, 29.3%, 78.2%, and 57.3%, respectively (Table 4).
When the diagnostic accuracy of 18F-FDG PET/CT was evaluated according to the suspicion of recurrence, all 15 patients with recurrence belonged to recurrence-suspected group. The sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of the 18F-FDG PET/CT in the recurrence-unsuspected group were 42.9%, 61.0%, 37.1%, 75.0%, and 57.8%, respectively. In contrast, there were 4 FDG uptakes at the stomach, regional lymph node and colon in patients without suspicion of recurrence. Seven patients had no FDG uptake. However, there was no recurrence in any of the 11 patients. None of unsuspected recurrence patients showed recurrence and the sensitivity of 18F-FDG PET/CT scans was undefined. The specificity, positive predictive value, negative predictive value, and diagnostic accuracy were 46.2%, 0.0%, 100.0%, and 53.8%, respectively.
Twenty-seven sites were suspicious for recurrences. Eighteen of the 27 sites were diagnosed as true recurrent lesions (Table 5). Eleven sites were detected only by contrast CT scan, including the intestine (n = 3), peritoneum (n = 4), pancreas (n = 1), bile duct (n = 1), ureter (n = 1), and ovary (n = 1) (Fig. 2).
Contrast CT scans showed a higher sensitivity (85.8%) and specificity (87.3%) than 18F-FDG PET/CT scans in detection of gastric cancer recurrence (Table 6).
When divided into two groups, one group without suspicion of recurrence on contrast CT and 18F-FDG PET/CT, and the other group with suspicion of recurrence on either contrast CT or 18F-FDG PET/CT, the sensitivity of combination rose to 95.2% and the specificity declined to 45.6% (Table 7).
Gastric cancer progresses by regional direct invasion, lymphatic spread, and hematogenous metastasis. The most commonly affected organs include the regional lymph nodes, bowel, liver, and peritoneum. Currently, we use endoscopy, contrast CT scans, and serum tumor markers to evaluate recurrence after curative gastrectomy. The contrast CT scan has a high sensitivity for other solid organ metastases, such as the liver and ovary, but a low diagnostic accuracy for lymphatic or peritoneal metastases. After gastrectomy and lymph node dissection for gastric cancer, anatomic structures may change, and a post-operative CT scan may have limited diagnostic value. Also, in peritoneal metastasis, other factors, such as the presence of ascites, location of metastasis, and mesenteric fat, may influence the interpretation of CT scan images.13
In this study, we assessed the diagnostic usefulness of 18F-FDG PET/CT scans in the evaluation of gastric cancer recurrences with clinical follow-up for more than 12 months to make sure any obscure lesions; 18F-FDG PET/CT scans had lower sensitivity and specificity than contrast CT scans. One reason for this is the histopathologic characteristics of gastric cancer. Adenocarcinoma, the most common pathologic type of gastric cancer, has low 18F-FDG-uptake.14,15 Furthermore, when taking cellular differentiation into account, poorly differentiated cells or signet ring cell adenocarcinoma progress more rapidly and has lower 18F-FDG-uptake than the well-differentiated cell type. In this study, unfortunately, well and moderately differentiated adenocarcinomas consisted of only 3.4% and 16.9% of cases. The rest of the cases were poorly differentiated, signet ring, or mucinous cell adenocarcinomas. Eighty two percent of cases had the Lauren classification diffuse type, and 14 of the 15 patients with recurrence had this same type in histologic features. Six of the 15 patents with recurrence had increased 18F-FDG-uptake, but none of five recurring signet ring cell carcinomas had FDG uptake. This may contribute to the low recurrence detection rate by PET/CT.
Another possible reason for lower sensitivity is the physiologic limitation of the gastrointestinal tract and peritoneum. It is difficult to differentiate tumorous lesions from non-tumorous lesions in the gastrointestinal tract because of the mucosal physiologic FDG uptake, as well as the inherent peristaltic movement.13 In our study, 18F-FDG PET/CT revealed a high false positive rate in the gastrointestinal tract. We noted 10 false positive cases that had FDG uptake in the remnant stomach. According to conventional protocols, luminal expansion was not sufficient during the examination and SUV could be over-estimated. Drinking water before the examination may help solve this problem and decrease the false positive rate.14 This method will partly improve the diagnostic values of 18F-FDG PET/CT. CT scans diagnosed 4 recurrent cases in the peritoneum, but 18F-FDG PET/CT did not detect any cases. Peritoneal metastasis does not make a lump in most cases. Instead, fine granulations are usually found. Contrast enhanced high resolution CT could detect thickened omentum and serrated peritoneum, but frequently combined ascites or fluid collection makes vague images of PET/CT worse.
The indication of 18F-FDG PET/CT study is another possible cause for lower sensitivity. One study about PET/CT showed that 75 of 105 patients (71.4%) had recurrence after the curative gastrectomy. It's sensitivity, specificity, positive predictive value, negative predictive value and accuracy were 75%, 77%, 89%, 55%, and 75%, respectively.7 Another study reported that 14 of the 23 patients (60.9%) were diagnosed with recurrence. The positive predictive value, negative predictive value and accuracy were 85.7%, 77.7%, and 82.6%, respectively.8 Another study reported that 20 of 33 patients (61%) had recurrence. The sensitivity, specificity, positive and negative predictive values were 70%, 69%, 78%, and 60%, respectively.10 Compared with those studies, our data showed a lower recurrence rate, lower sensitivity and lower diagnostic accuracy. The low recurrence rate most likely resulted from extended indication of the PET/CT. We believe that the lower diagnostic power in our study might have been caused by the low recurrence rate.
This explanation was supported by one study. Patients were grouped according to suspicion of recurrence. Recurrence was already suspected by other imaging modalities and in this group (Group A) recurrence of gastric cancer was confirmed in 31 (67%) of the 46 patients, another group (Group B) was suspected of recurrence by only tumor markers and recurrence was confirmed in 11 (58%) of the 19 patients, the other group (Group C) underwent a PET scan without evidence of recurrence and recurrence was confirmed in 2 (7%) of 27 patients. The sensitivity, specificity and diagnostic accuracy of PET for recurrence were 81%, 87% and 83%, respectively, in Group A; 73%, 88% and 79%, respectively, in Group B; and 50%, 88% and 85%, respectively, in Group C. The sensitivity of PET/CT was lower when recurrence was not suspected.11
Therefore, it is inadvisable for patients with a low probability of recurrence to perform 18F-FDG PET/CT because of its low diagnostic value.
In our study, 11 patients were suspected to have no recurrence. None of them showed recurrence and measurement of diagnostic values of PET/CT seemed insignificant.
Recently published data reported that additional PET/CT on CT scan did not increase diagnostic accuracy in detection of recurred gastric cancer. Thirty eight of 52 patients (73.1%) were confirmed to have recurrence. The sensitivity was 68.4% for PET/CT and 89.4% for contrast CT. The specificity was 71.4% and 64.2%, respectively. The final diagnostic accuracy of PET/CT was 42.8%. They concluded that additional PET/CT on contrast CT did not increase diagnostic accuracy in detection of recurred gastric cancer.9
On the other hand, 18F-FDG PET/CT also has the ability to detect other primary lymph nodes or tumors. In this study, we found 3 primary malignant sites in 2 of the 89 patients (2.2%), one in the thyroid and two in the lungs. There were several reports of incidental primary cancer by 18F-FDG PET/CT. According to one of these studies, 272 of 2,219 cancer patients (12%) had findings of FDG uptake. An invasive work-up was performed on 49% (133/272) of these patients. A second primary malignancy was found in 41 patients (31%). The common sites for a proven second primary malignancy were the lungs (n = 10), breast (n = 7), and colon (n = 5).16 Another report examined 1,912 patients and a second primary cancer was detected in 1.2% among suspected 4.1% lesions. Proven second primary malignancies were found in the lungs (n = 7), thyroid (n = 6), colon (n = 4), breast (n = 2), esophagus (n = 2), bile duct (n = 1), and the head and neck (n = 1).17 This suggests that the detection of unexpected second primary malignancy may be another important role of PET/CT.
There were several limitations on our study. The first was the small sample size, which may have produced a statistically limited value. The second was the retrospective design, so the suspicion of recurrence and proper indication of 18F-FDG PET/CT studies were not well defined.
In conclusion, the 18F-FDG PET/CT scan alone is an insufficient diagnostic method in the evaluation of recurrence after curative gastrectomy due to the low sensitivity and specificity, and demonstrated an even lower rate of accuracy than the contrast CT scan alone. When 18F-FDG PET/CT scan combined with a CT scan diagnosed recurrence, the sensitivity improved a little but the specificity declined. Therefore, additional 18F-FDG PET/CT on contrast CT scan is an inadvisable method of detecting gastric cancer recurrence.
Figures and Tables
Fig. 1
A case of recurring gastric cancer detected by FDG-uptake on PET/CT. This 57 year-old man showed no suspicion of recurrence on the abdomino-pelvic CT scan. However, on the whole body PET/CT scan, there was a high FDG-uptake on the left supraclavicular lymph node. This lesion was confirmed as distant nodal metastasis by biopsy. FDG, fluorodeoxyglucose; PET, positron emission.
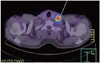
Fig. 2
A case of recurrent gastric cancer detected by CT scan but showing no FDG-uptake on PET/CT. (A) This 52 year-old woman showed peritoneal carcinomatosis and right ovarian enlargement with septated cystic mass on CT scan. (B) However, on the 18F-FDG PET/CT scan, there was no abnormal FDG-uptake. The recurrence was confirmed by peritoneal fluid cytology. FDG, fluorodeoxyglucose; PET, positron emission.

Table 4
Diagnostic Accuracy of 18F-FDG PET/CT in Detecting Postoperative Recurrence after Curative Gastrectomy

Table 6
Diagnostic Accuracy of CT Scan in Detecting Postoperative Recurrence after Curative Gastrectomy

References
1. Schwarz RE, Zagala-Nevarez K. Recurrence patterns after radical gastrectomy for gastric cancer: prognostic factors and implications for postoperative adjuvant therapy. Ann Surg Oncol. 2002. 9:394–400.

2. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000. 87:236–242.

3. Lim JS, Yun MJ, Kim MJ, Hyung WJ, Park MS, Choi JY, et al. CT and PET in stomach cancer: preoperative staging and monitoring of response to therapy. Radiographics. 2006. 26:143–156.

4. Habermann CR, Weiss F, Riecken R, Homarpisheh H, Bohnacker S, Staedtler C, et al. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology. 2004. 230:465–471.

5. Kuntz C, Herfarth C. Imaging diagnosis for staging of gastric cancer. Semin Surg Oncol. 1999. 17:96–102.

6. Halvorsen RA Jr, Yee J, McCormick VD. Diagnosis and staging of gastric cancer. Semin Oncol. 1996. 23:325–335.
7. Park MJ, Lee WJ, Lim HK, Park KW, Choi JY, Kim BT. Detecting recurrence of gastric cancer: the value of FDG PET/CT. Abdom Imaging. 2009. 34:441–447.

8. Sun L, Su XH, Guan YS, Pan WM, Luo ZM, Wei JH, et al. Clinical role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in post-operative follow up of gastric cancer: initial results. World J Gastroenterol. 2008. 14:4627–4632.

9. Sim SH, Kim YJ, Oh DY, Lee SH, Kim DW, Kang WJ, et al. The role of PET/CT in detection of gastric cancer recurrence. BMC Cancer. 2009. 9:73.

10. De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging. 2002. 29:525–529.

11. Nakamoto Y, Togashi K, Kaneta T, Fukuda H, Nakajima K, Kitajima K, et al. Clinical value of whole-body FDG-PET for recurrent gastric cancer: a multicenter study. Jpn J Clin Oncol. 2009. 39:297–302.

12. Sobin LH, Wittekind CH. TNM classification of malignant tumors. International union against cancer (UICC). 2002. 6th ed. New York: Wilwy-Liss.
13. Yun M, Kim BI. Roles of F-18 FDG PET or PET/CT for the evaluation of gastrointestinal malignancies. Korean J Gastroenterol. 2006. 48:378–387.
14. Yun M, Choi HS, Yoo E, Bong JK, Ryu YH, Lee JD. The role of gastric distention in differentiating recurrent tumor from physiologic uptake in the remnant stomach on 18F-FDG PET. J Nucl Med. 2005. 46:953–957.
15. Nunez RF, Yeung HW, Macapinlac H. Increased F-18 FDG uptake in the stomach. Clin Nucl Med. 1999. 24:281–282.

16. Beatty JS, Williams HT, Aldridge BA, Hughes MP, Vasudeva VS, Gucwa AL, et al. Incidental PET/CT findings in the cancer patient: how should they be managed? Surgery. 2009. 146:274–281.

17. Ishimori T, Patel PV, Wahl RL. Detection of unexpected additional primary malignancies with PET/CT. J Nucl Med. 2005. 46:752–757.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download