This article has been corrected. See "Erratum to "Effects of Multiple Drilling on the Ischemic Capital Femoral Epiphysis of Immature Piglets" by Gong SY, et al. (Yonsei Med J 2011;52:809-17.)" in Volume 53 on page 240.
Abstract
Purpose
This study investigated the effects of multiple drilling on the immature capital femoral epiphysis following ischemic injury in a piglet model.
Materials and Methods
Ischemic necrosis of capital femoral epiphysis was induced bilaterally in 12 piglets using a cervical ligation method. Three weeks later, medial, central, and lateral 3 drill holes were made on the left femoral head using 0.062" K-wire. At 3, 6, 9, and 12 weeks following the multiple drilling, femoral heads were harvested from each three piglets. On histologic examination, percent of revascularization, percent of osteoblast surface, capital femoral epiphyseal quotient and proximal femoral growth plate height were evaluated. Untreated right femoral heads served as control.
Results
While percent of revascularization of left capital femoral epiphysis with multiple drilling was significantly higher than untreated control side (p<0.001), percent of osteoblast surface, capital femoral epiphyseal quotient and proximal femoral growth plate height showed no significant difference.
Conclusion
This study indicates that multiple drilling could promote revascularization of ischemic capital femoral epiphysis, and multiple drilling does not appear to produce bony physeal bars at short-term, if using small diameter drill. However, multiple drilling alone does not seem to prevent femoral head deformity or to promote new bone formation.
Legg-Calve-Perthes disease is one of the most common diseases involving hip joint in growing children.1 The etiology is still obscure, however, many previous clinical and experimental studies have suggested that ischemic injury on the immature capital femoral epiphysis plays a major role in pathogenesis.2
Contrary to the osteonecrosis of the femoral head in adult, LCP disease accompanies remodeling process during its natural course.3 During remodeling process, dead bone is replaced with living bone, and the deformed femoral head can remodel within the acetabular socket, thereby providing the chance to restore the spherical congruency of the hip joint.4 Many surgical or non-surgical treatments focus on so called "containment" of the femoral head into the acetabular socket to facilitate the restoration of spherical congruency during the remodeling period. Therefore, if properly treated, the prognosis of LCP disease is thought to be better than that of osteonecrosis of the femoral head in adult, which results in progressive head collapse and subsequent degenerative arthritis of the hip joint. However, the remodeling capacity of the femoral head depends on the extent of the disease-involvement and the age at the onset of the disease. The patients with extensive involvement and/or near the skeletal maturity would have little remodeling capacity. "Containment" treatment does not consistently produce spherical femoral head in the older patients,5-7 which results in deformed femoral head and subsequent early degenerative arthritis of the hip joint. Therefore, there have been efforts to develop effective treatment modalities other than "Containment" for these patients with poor prognosis.7
Multiple drilling is a treatment method that does not rely on containment. The rationale of multiple drilling is to provide conduits for revascularization to the ischemic femoral head so as to prevent further ischemic injury and facilitate healing of necrotic bone. Indeed, several clinical studies have shown promising outcomes after multiple drilling on the osteonecrosis of the femoral head in adult, supporting the efficacy of multiple drilling on ischemic femoral head,8 nevertheless, multiple drilling has rarely been performed on LCP disease.9,10 Although LCP disease and osteonecrosis after femoral neck fracture were the first reported cases in which multiple drilling had been performed,11 many surgeons are concerned about possible damage to the proximal growth plate, and the efficacy of multiple drilling on ischemic immature femoral head has not yet been reported.
The purpose of this study was to evaluate the effects of multiple drilling on the ischemic immature femoral head using a piglet model. We investigated whether multiple drilling could increase revascularization on the ischemic femoral head and prevent collapse of the femoral head. We also investigated the effect of multiple drilling on the proximal growth plate.
The study design was approved by the Institutional Animal Care and Use Committees. Twelve female piglets, five to six weeks old and weighing 5 to 6 kg, were used. Ischemic injury was surgically induced to femoral capital epiphyses on both sides. Three weeks later, multiple drilling was performed on the left femoral head, while the right femoral head was left undone to serve as a control. Histological evaluation was performed at 3, 6, 9 and 12 weeks after multiple drilling.
Ischemic insult to the capital femoral epiphysis was induced using cervical ligation method, as described by Kim, et al.12 Under the general anesthesia, piglets were laid on the table in the lateral decubitus position. Lateral transverse skin incision was made over the hip joint. The gluteus and underlying short abductor muscles were split and retracted aside. After capsulotomy, ligamentum teres was severed blindly using curved scissors. With use of curved passer instrument, two No. 2 Ethibond (ETHIBOND EXCEL Polyester Suture, Ethicon Inc., Somerville, NJ, USA) sutures were placed around the femoral neck and were manually tied as tightly as possible (Fig. 1). Then, 1 mm drill hole was made from the cartilaginous articular surface to the cancellous bone of femoral capital femoral epiphysis, and the establishment of ischemia on the femoral head was confirmed by observing that no blood was coming out from the drill hole. Wound was closed layer by layer. Surgery was performed on both sides at the same time.
Multiple drilling was performed three weeks after cervical ligation. Under the general anesthesia, piglets were laid on the table in the prone position. About 2 cm-sized skin incision was made along adductor muscle, and then medial femoral neck was exposed with blunt dissection through adductor intermuscular plane. With the guidance of image intensifier, drilling was performed three times with 0.062" Kirschner wires at 2,000 revolutions per minute. Using the same entry portal at medial cortex of femoral neck, wire was inserted aiming at medial, central, and lateral one third of capital femoral epiphysis through proximal growth plate (Fig. 2).
Bilateral femora were harvested 3, 6, 9, and 12 weeks after multiple drilling (6, 9, 12, and 15 weeks after cervical ligation). Before the process for histologic sections, antero-posterior radiographs were taken with bilateral femora after harvest. The radiographic film was placed directly under the bone to minimized magnification effects. For histologic evaluation, a representative section from the central load-bearing region for the femoral head was analyzed. The region was chosen because it is the region of maximal femoral head height and diameter, corresponding to the outline of the femoral head seen on the AP radiographs.13 Specimens were fixed with 10% formalin solution at 4℃ for 7 days, and then decalcified with 5% AgNO solution at 4℃ for 3 to 4 days. One cm-thick-slices were made through the central portion of the femoral heads with maximum diameter in the coronal plane and embedded in paraffin, and then further sectioned at a thickness of 7 um. The mounted sections were stained with hematoxylin and eosin. For histomorphologic evaluation, photographs of each section were taken with Nikon E800 microscope (Nikon USA, Melville, NY, USA) at various magnifications, and the measurement was made with use of Image-Pro Plus (version 4.5; Media Cybernetics, Silver Spring, MD) software program.
To assess the revascularization effects of multiple drilling on ischemic capital femoral epiphysis, percent of revascuralization within the central region of the femoral head was determined. Percent of revascularization is the percent area containing fibrovascular repair tissue divided by the total capital femoral epiphysis area.13 Measurements were made at ×4 magnification.
To evaluate the effect of multiple drilling on the new bone formation as a repair process after an ischemic injury, percent of osteoblast surface was measured. Percent of osteoblast surface is the percent trabecular surface area covered with cuboidal-shaped osteoblasts.14 Measurements were made at ×20 magnification. Scanning the whole capital femoral epiphysis under a microscope, measurement was made in the area of repair away from the chondro-osseous junction so that the primary spongiosa would not be included in the analysis. The necrotic areas of the femoral head showing absence of osteoblasts were not measured.13
To evaluate the effect of multiple drilling on the growth plate, the thickness of the femoral neck growth plate was measured. The thickness of growth plate was measured from the edge of the reserve zone to the last hypertrophic chondrocyte in the transverse cartilage septum before vascular ingrowth.16 The thicknesses was measured at the medial, middle and lateral thirds of the growth plate and averaged (Fig. 4).
Statistical analysis was accomplished using SPSS statistical package (version 13.0; SPSS Inc., Chicago, IL, USA). A two-way analysis of variance (ANOVA) was used, with treatment (control versus multiple drilling) and time (time of harvest after multiple drilling) as the grouping variables. The level of significance was set at p<0.05. All data are presented as mean±standard deviation.
All the specimens showed growth arrest of the bony epiphysis, empty lacunae in the trabecular bone, necrotic changes in the marrow space, and the presence of fibrovascular repair tissue, which indicated that ischemic osteonecrosis on capital femoral epiphysis was successfully induced in all the study animals (Figs. 5 and 6).
The histopathological changes on the capital femoral epiphysis of the control side, where ischemic insult was induced without multiple drilling, were very similar to those previously described by Kim, et al.12 The specimens harvested six weeks after ischemic insult showed oval shaped deformity losing normal sphericity of the femoral heads. On examination with high power, the central portion of capital femoral epiphysis was filled with fibrovascular tissue instead of the bony trabeculae and marrow fat cells. Some bony trabeculae still remained in the central marrow spaces of capital femoral epiphysis, however, lacunae of trabeculae were empty and the trabeculae were surrounded by fibrovascular tissues without osteogenic cell linings. In this study, we failed to observe the appearance of new accessory ossification centers in the capital femoral epiphysis, however, there were evident new bone formation on the growth cartilage surrounding the secondary ossification center of the capital femoral epiphysis. The gross appearance of the capital femoral epiphysis was like an eggshell that had peripheral new bone trabeculae and central empty space filled with fibrovascular tissue. The specimens harvested 9, 12, and 15 weeks after ischemic insult showed similar histopathologic changes. The thickness of peripheral bony trabeculae which were formed by the growth cartilage surrounding the secondary ossification center of capital femoral epiphysis increased, however, fibrovascular tissue without any evidence of new bone formation still remained in the central marrow space of the capital femoral epiphysis. Taken all together, the histopathologic changes after ischemic insult on capital femoral epiphysis could be summarized as permanent ischemic necrosis without bony healing in the central marrow space and temporary ischemic necrosis followed by restored enchondral ossification of epiphyseal growth cartilage in the periphery (Fig. 7). Revascularization with fibrovascular tissue invasion was observed in the periphery first, and then to the central marrow space over time, preceding the restoration of enchondral ossification of the growth cartilage surrounding the secondary ossification center of the capital femoral epiphysis. Despite the new bone formation at peripheral growth plate, femoral head deformity progressed with central collapse (Fig. 7).
On the specimens harvested from the treatment side, disruptions of cervical growth plate, which were made by multiple drilling, were observed. Since we took a small coronal piece from the center of femoral head to make histologic sections, the sections from only 7 of 12 specimens included drill holes in them. One of the striking changes after multiple drilling was rapid revascularization to the whole capital femoral epiphysis. While fibrovascular invasion started at the periphery, on the multiple drilling-untreated femoral head of the control side, and then progressed to the central region overtime, abundant fibrovascular tissues invasion from the cervical metaphysis to the whole capital femoral epiphysis through disruption of metaphyseal growth plate which was made by drilling was observed on the treatment side (Fig. 8). Compared by percent of revascularization, revascularization was significantly faster after multiple drilling (Table 1), although revascularizationof ischemic femoral head progressed with time in both groups.
Despite rapid revascularization, bony healing process after multiple drilling was not different from those without multiple drilling. In the central marrow space of capital femoral epiphysis, necrotic bony trabeculae and marrow cells were replaced by fibrovascular tissue without new bone formation. New bone formation took place only at the growth cartilage surrounding the secondary ossification of the capital femoral epiphysis by restored enchondral ossification. Percent of osteoblast surface increased with time, however, showed no significant difference between femoral heads of both sides (Table 1) (Fig. 9), indicating that multiple drilling did not facilitate new bone formation on the central marrow space of the ischemic capital femoral epiphysis. Lack of bony healing in the central region led central collapse, which resulted in gross femoral head deformity on the treatment side as well. The degree of femoral head deformity, measured by epiphyseal quotient increased with time, however, we could find no significant difference between femoral heads of both sides (Table 1). In other words, multiple drilling could not prevent central collapse of ischemic capital femoral epiphysis (Fig. 9).
Disruption area of cervical metaphyseal growth plate, which was made by multiple drilling, served as revascularization channel with fibrovascular tissue filled inside. Although we could evaluate the fate of these drill holes on the growth plate in only 7 of 12 specimens, no bony bridge formation within the drill holes was noted. The thickness of cervical metaphyseal growth plate showed no significant difference between femoral heads of both sides (Table 1).
Thus, on histologic examinations, we could not find any adverse effects of multiple drilling on cervical metaphyseal growth plate.
The strategy of multiple drilling as a treatment modality for the ischemic capital femoral epiphysis is based on a distinct vascular anatomy of the immature femoral head. In children, the proximal femoral growth plate imposes a barrier to the intraosseous blood flow from the metaphysis to epiphysis, so that the capital femoral epiphysis depends exclusively on extraosseous retinacular vessels.17 The retinacular vessels arise from the vascular ring around the base of neck, ascend along the surface of the neck, and enter peripheral to the base of capital femoral epiphysis, which render the capital femoral epiphysis more vulnerable to ischemic insult.17 Revascularization after ischemic event takes more time, especially on central portion, because revascularization also depends on the peripheral extraosseous retinacular vessels. Clinically slow revascularization may prolong the duration of disease course, and the collapse of central ischemic epiphysis during slow revascularization process may endanger the regenerated vasculatures from the periphery to cause another round of ischemic event.18
The aim of multiple drilling is to make a channel on the growth plate barrier, so that the intraosseous blood flow from the metaphysis may supply the ischemic epiphysis cross the growth plate.19 In this study, we could observe that after multiple drilling, the abundant fibrovascular repair tissue came from the metaphysis crossing the growth plate through the drill hole and spread radially to the capital epiphysis. These intraosseous vasculatures induced rapid revascularization to the ischemic capital femoral epiphysis, suggesting that multiple drilling could serve as one treatment option for rapid revascularization in LCP disease.
One of the concerns about multiple drilling is a possible damage to the growth plate that has already suffered from ischemic insult. However, considering the fact that the candidates for this procedure would be the older patients with poor regeneration capacity and/or extensive disease involvement, the adverse effects of multiple drilling on the growth plate may not be clinically significant. Moreover, in this study, although we did not evaluate the long term effect till growth maturity, the height of growth plate showed no difference after multiple drilling or we could not observe any bone bridge formation till 12 weeks after multiple drilling. These observations are compatible to a previous report which showed that less than 7% of drill injury to the growth plate did not cause growth disturbance.20
Despite the rapid revascularization, we could not observe significant changes in amount of new bone formation at the central marrow space of the capital femoral epiphysis or in the degrees of femoral head collapse after multiple drilling. Lack of new bone formation in the central marrow space of capital epiphysis is not a new finding. In the piglet model of ischemic necrosis, Kim, et al. observed predominant osteoclastic bone resorption during repair process without following new bone formation.10-14 They regarded the early bone loss, the lack of new bone formation, and the persistence of fibrovascular tissue in the area of bone resorption as key pathologies which compromise the structural integrity of the femoral head and produce progressive femoral head collapse. In this study, we could observe new bone formation by restored enchondral ossification at the growth cartilage surrounding the secondary ossification center. However, there was no appositional new bone formation noted in the central marrow space of the capital femoral epiphysis. This repair process in the piglet model is different from the healing process in adult animal model of osteonecrosis.21 In the rabbit model of adult ischemic necrosis, the invasion of marrow vascular spaces of the "dead" portion of femoral head by the proliferative mesenchymal cells and capillaries from adjacent "living"metaphysis of femoral neck was observed at the early stage of repair process. These mesenchymal cells differentiated into osteoblastic lineage cells and led to appositional new bone formation, which replaced the necrotic bone with appositional new bone. It is still not clear why new bone formation does not take place in the ischemic capital femoral epiphysis. One of the possible explanation is the existence of the proximal femoral growth plate barrier, which does not allow the osteoprogenitor cells from the adjacent metaphysis of femoral neck to invade the ischemic capital epiphysis. We expected that the recruitment of the proliferative mesenchymal cells from the metaphysis below the growth plate through the drill hole on the growth plate would facilitate new bone formation. However, there was no increased new bone formation after multiple drilling, even though we could observe rapid revascularization. These observations suggested that rapid revascularization alone does not lead to increased new bone formation. Although further studies are required, it may be related to the biological milieu of the ischemic capital femoral epiphysis, which precludes new bone formation.
We acknowledge a number of limitations in the present study. First, the number of animals used in this study was relatively small. Therefore, the present results should be interpreted with caution due to the potential for type II error. Second, the osteoclastic activity after multiple drilling was not evaluated on histologic sections in this study. The result of this study showed that multiple drilling promoted rapid revascularization, but not new bone formation. Therefore, if rapid revascularization after multiple drilling promotes osteoclastic activity and bone resorption, multiple drilling alone may aggravate femoral head collapse of the patients with LCPD, which would be a potential limitation of its clinical application. Although this study showed no significant difference in epiphyseal quotients between control and multiple drilling groups, further study is required to determine the effect of multiple drilling on osteoclastic activity and bone resorption. Third, to evaluate the adverse effect of multiple drilling on the metaphyseal growth plate, we measured growth plate thickness. This is an easy and simple method, however, it may not be a sensitive indicator to evaluate the function of the growth plate and the observation was made only for 12 weeks after the procedure. Although the results of this study suggest that the short-term adverse effects on the metaphyseal growth plate after multiple drilling may not be prominent, the long-term consequences are still unclear.
The results of this study showed that multiple drilling facilitates revascularization of ischemic capital femoral epiphysis. However, multiple drilling alone does not increase new bone formation and does not prevent the femoral head deformity. The damage to the proximal femoral growth plate may be limited if a small size drill is used. Further studies are needed to assess the functional status of the growth plate over a long term.
Figures and Tables
Fig. 1
An experimental model of ischemic necrosis of the capital femoral epiphysis using a cervical ligation. After a longitudinal skin incision over the left hip joint under sterile condition, a partial capsulotomy was performed and the ligamentum teres was transected. Two ethibond sutures were placed around the neck and hand-tied using slip-proof ligation technique as tightly as possible to disrupt the ascending cervical vessels supplying the capital femoral epiphysis (A). To evaluate the position of cervical ligation, stainless steel wire was used in pilot study and the postoperative radiograph showed proper position of cervical ligation wires (B).

Fig. 2
Three weeks following ischemic insult, drilling was performed using 0.062" K-wire and a motorized drill under fluoroscopic control. Medial, central, and lateral three drill holes were made from medial side of cervical neck through the proximal femoral growth plate to the capital femoral epiphysis.
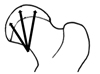
Fig. 3
Capital femoral epiphyseal quotient was defined as height of osseous capital epiphysis divided by maximum transverse diameter.
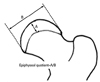
Fig. 4
After staining with hematoxylin and eosin, the thickness of the growth plates was measured. The growth plate was divided into three regions on coronal sections (medial, middle, and lateral thirds, and dot lines show borders of each region) and the thicknesses were measured along the direction of enchondral ossification (axis of arrows) at each region and averaged.

Fig. 5
Photomicrographs of normal femoral head of immature piglet. (A) Polycut section view (H&E, ×4). (B) Higher magnification of the growth cartilage surrounding the secondary ossification center shows normal columnar pattern (H&E, ×10). (C) Higher magnification of the growth plate cartilage of the metaphyseal physis shows normal cellular zones including reserve, proliferative and hypertrophic zones. Vascular invasion of terminal hypertrophic chondrocytes and primary spongiosa formation in the metaphysis, indicative of enchondral ossification, are evident (H&E, ×10).
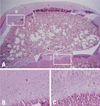
Fig. 6
Photomicrographs of the femoral head of immature piglet at two weeks following induction of ischemia. (A) Polycut section view (H&E, ×4). (B) The growth cartilage surrounding the secondary ossification center shows empty lacunae at growth plate cartilage, which indicates establishment of ischemic insult to the capital femoral epiphysis (H&E, ×10). (C) The growth plate of the metaphyseal physis also shows disarray of normal pattern of columnization and necrosis after the ischemic damage (H&E, ×10).
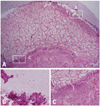
Fig. 7
Photomicrographs of the femoral head of immature piglet at fifteen weeks following induction of ischemia. (A) Polycut section view (H&E, ×1). (B) The growth cartilage surrounding the secondary ossification center shows complete regeneration of normal columnar pattern and enchondral ossification (H&E, ×10). (C) In the central area of the secondary ossification center, necrotic bony trabeculae remnants intermixed with fibrovascular scar tissue are noted, and there is no evidence of new bone formation at all (H&E, ×10).
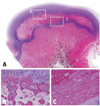
Fig. 8
Photomicrographs of the femoral head of immature piglet at three weeks following multiple drilling. (A) Polycut section view (H&E, ×1). (B and C) The centro-medial and centro-lateral junctions of the proximal femoral growth plate are disrupted by drilling. From cervical metaphysis to the secondary ossification center through transphyseal hole, abundant fibrovascular invasion is observed (H&E, ×4).
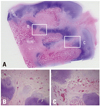
Fig. 9
Photomicrographs (H&E, ×1) and antero-posterior radiographs of the femoral head of immature piglet at twelve weeks following drilling. Although multiple drilling provided increased revascularization on the ischemic capital femoral epiphysis, there was no significant difference in head collapse or proximal femoral growth plate height between untreated control group (A and C) and multiple drilling group (B and D).
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (KRF-2007-331-E00124).
References
1. Rowe SM, Jung ST, Lee KB, Bae BH, Cheon SY, Kang KD. The incidence of Perthes' disease in Korea: a focus on differences among races. J Bone Joint Surg Br. 2005. 87:1666–1668.
2. Thompson GH, Salter RB. Legg-Calvé-Perthes disease. Current concepts and controversies. Orthop Clin North Am. 1987. 18:617–613.
4. Lloyd-Roberts GC, Catterall A, Salamon PB. A controlled study of the indications for and the results of femoral osteotomy in Perthes' disease. J Bone Joint Surg Br. 1976. 58:31–36.

5. Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part II: Prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am. 2004. 86-A:2121–2134.
6. Willett K, Hudson I, Catterall A. Lateral shelf acetabuloplasty: an operation for older children with Perthes' disease. J Pediatr Orthop. 1992. 12:563–568.
7. Joseph B, Mulpuri K, Varghese G. Perthes' disease in the adolescent. J Bone Joint Surg Br. 2001. 83:715–720.

8. Song WS, Yoo JJ, Kim YM, Kim HJ. Results of multiple drilling compared with those of conventional methods of core decompression. Clin Orthop Relat Res. 2007. 454:139–146.

9. Pohl J. [A contribution to covered drilling in perthes' disease]. Arch Orthop Unfallchir. 1964. 56:661–663.
11. Bozsan EJ. A new treatment of intracapsular fractures of the neck of the femur and Calve-Legg-Perthes' disease. J Bone Joint Surg Am. 1932. 14:884–887.
12. Kim HK, Su PH, Qiu YS. Histopathologic changes in growth-plate cartilage following ischemic necrosis of the capital femoral epiphysis. An experimental investigation in immature pigs. J Bone Joint Surg Am. 2001. 83-A:688–697.
13. Kim HK, Morgan-Bagley S, Kostenuik P. RANKL inhibition: a novel strategy to decrease femoral head deformity after ischemic osteonecrosis. J Bone Miner Res. 2006. 21:1946–1954.

14. Kim HK, Randall TS, Bian H, Jenkins J, Garces A, Bauss F. Ibandronate for prevention of femoral head deformity after ischemic necrosis of the capital femoral epiphysis in immature pigs. J Bone Joint Surg Am. 2005. 87:550–557.

15. Heyman CH, Herndon CH. Legg-Perthes disease; a method for the measurement of the roentgenographic result. J Bone Joint Surg Am. 1950. 32:767–778.
16. Kienzle L. [Therapy of coxa vara epiphysaria and Perthes' disease by the Beck's drilling-operation]. Z Orthop Ihre Grenzgeb. 1953. 83:270–275.
17. Chung SM. The arterial supply of the developing proximal end of the human femur. J Bone Joint Surg Am. 1976. 58:961–970.

18. Atsumi T, Kuroki Y, Yamano K. A microangiographic study of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1989. 186–194.

19. Dahners LE, Hillsgrove DC. The effects of drilling on revascularization and new bone formation in canine femoral heads with avascular necrosis: an initial study. J Orthop Trauma. 1989. 3:309–312.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download