Abstract
Purpose
Recently, several clinicians have reported the advantages of simplicity and cosmetic satisfaction of absorbable mesh insertion. However, there is insufficient evidence regardint its long-term outcomes. We have investigated the surgical complications and postoperative examination from the oncologic viewpoint.
Materials and Methods
From February 2008 to March 2009, 34 breast cancer patients underwent curative surgery with absorbable mesh insertion in Samsung Medical Center. Patient characteristics and follow up results including complications, clinical and radiological findings were retrospectively investigated.
Results
The mean age of the study population was 50.1±8.9 years old (range 31-82) with a mean tumor size of 3±1.8 cm (range 0.8-10.5), and the excised breast tissue showed a mean volume of 156.1±99.8 mL (range 27-550). Over the median follow-up period of 18±4.6 months (range 3-25), mesh associated complications, including severe pain or discomfort, edema, and recurrent fluid collection, occurred in nine patients (26.5%). In three cases (8.8%), recurrent mastitis resulted in mesh removal or surgical intervention. In the postoperative radiologic survey, the most common finding was fluid collection, which occurred in five patients (16.1%), including one case with organizing hematoma. Fat necrosis and microcalcifications were found in three patients (9.7%).
Conclusion
Absorbable mesh insertion has been established as a technically feasible, time-saving procedure after breast excision. However, the follow-up results showed some noticeable side effects and the oncologic safety of the procedure is unconfirmed. Therefore, we suggest that mesh insertion should be considered only in select cases and should be followed-up carefully.
Absorbable surgical mesh insertion has recently been introduced for volume replacement after partial mastectomy. The idea is that the absorbable polyglycolic acid mesh may enable the breast to maintain its shape after excision of the diseased tissue by forming reactive fluid with capsulation via creation of peri-fibrosis reand formation of granulation tissue at the peripheral area of the mesh, with gradual absorption of foreign material.1,2 In general, oxidized regenerated cellulose is also used to prevent adhesion between the skin and the polyglycolic acid mesh.1 According to the previous reports, the absorbable mesh insertion is a simple, time-sparing technique.1
In breast cancer surgery, breast conservation has become the standard therapy, with better patient satisfaction and comparable long-term outcomes than total mastectomy.3 Still, surgeons and patients have continuously tried to achieve the oncologic goals of complete tumor excision with enough margins as well as good aesthetic outcomes with the remaining breast.4 Especially for Asian women with small dense breasts, volume replacement after partial excision has been one of the major concerns and one of the great obstacles for partial mastectomy at the same time. Preliminary use of mesh suggested it as an ideal alternative for volume replacement.
Since 2005, when it was first introduced, many clinicians have reported positive cosmetic results with a comparably lower rate of complications at a minimal cost.5 Also, some studies with questionnaires have demonstrated comparable cosmetic satisfaction of patients with breast malignancies or benign breast diseases.1,6,7 However, these results have been limited to short-term outcomes of less than one year,7,8 and long-term outcomes have not been fully elucidated from the viewpoint of post-operative surveillances and oncologic safety. The aim of this study is to review the immediate and the long-term post-operative outcomes over a year after mesh insertion.
From February 2008 to March 2009, 34 breast cancer patients received partial mastectomy with axillary procedure, such as sentinel lymph node biopsy or axillary lymph node dissection, followed by insertion of absorbable mesh (Polyglactic 910, Vicryl®) at Samsung Medical Center, Sungkyunkwan University School of Medicine. The electronic medical records of these patients were retrospectively reviewed. Patient characteristics and follow-up results including complications, aesthetic outcomes, and clinical and radiological findings were investigated.
The oncologic surgical procedure including partial mastectomy and axillary lymph node excision with either sentinel lymph node biopsy or axillary lymph node dissection were performed in a routine manner. After confirmation of complete excision of the tumor by intraoperative frozen biopsy and meticulous bleeding control, the breast is irrigated with warm distilled water 2 to 3 times, on equal terms with general breast surgery. The absorbable mesh is designed and manipulated with absorbable sutures into the breast as previously reported. To prevent adhesion between mesh and the tissue, the mesh is sealed up with oxidized regenerated cellulose (Interceed®, Ethicon, Somerville, USA). The sector-shaped folded mesh, wrapped in the cellulose complex, is put into the defect of the breast and secured with a few stitches of absorbable suture to prevent rotation.
A drain is not inserted, because the absorption of the mesh via hydrolysis, reactive fluid formation, granulation reaction interferes with mechanical drainage. Intravenous (IV) antibiotics are injected within one hour prior to the operation. Postoperatively, after two or three days of IV antibiotics, oral antibiotics are prescribed for less than five days.
Post-operative survey included a medical record review, patient interview, and investigation of radiologic findings. Mammography and ultrasound were used for the postoperative radiologic survey.
The mean age was 50.1 years old (range 31-82) with a mean tumor size of 3±1.8 cm (range 0.8-10.5), and the excised breast tissue showed a mean volume of 156.1±99.8 mL (range 27-550). The upper outer quadrant was the most common tumor site (Table 1). Invasive ductal carcinoma was found in 25 patients (73.5%), followed by ductal carcinoma in site in 6 patients (17.7%), including 2 cases of invasive lobular carcinoma and one case of invasive tubular carcinoma. Most of the patients were early breast cancer patients but three (8.8%) were stage III. Consequent adjuvant hormonal or chemotherapy was undertaken in the standard manner according to the patient's disease stage. All but two patients refused to have radiation therapy.
During the median follow-up period of 18±4.6 months (range 3-25), mesh associated complications, including severe pain, discomfort and edema, occurred in nine patients (29.4%). Among these nine patients, mesh removal was inevitable in three cases (8.8%) of serious complications, one because of recurrent mastitis and two because of wound dehiscence. In the other six cases, there were symptoms of persistent pain, skin contraction, skin color change, and repeated seroma formation.
Two patients (5.9%) were lost during follow-up. One of these patients complained that she had persistent pain, which slowly improved with conservative management. Because she argued that she had general weakness due to her age (84), hospital visits were discontinued. The other patient developed severe mastitis two days after the first cycle of adjuvant chemotherapy, so the mesh was removed after 45 days of initial operation. This patient was referred to a local hospital due to accessibility problems from her residence to the hospital and follow up radiologic exam or clinical course was not obtained after mesh removal. These two patients were excluded from the imaging surveillance analysis.
During postoperative surveillance with mammography, heterogeneous changes were often found, such as parenchymal changes, ranging from simple skin dimpling or post operative parenchymal deformity, to speculated or irregular formation of mass densities (Fig. 1A). Five patients (14.7%) presented with findings with significant alert. Microcalcifications at the operation site suggesting fat necrosis were noted in three cases (8.8%), and an increase in the number of microcalcifications was found in two patients (6.9%) (Fig. 1B). The other two patients (6.9%) presented with mass-like densities (fluid collection), and reticular density (foreign body shadow).
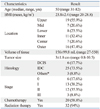
Ultrasound examination also showed problematic results. There were not only simple well-capsulated fluid collections or typical ovoid benign-looking mass formations, but also irregularly demarcated mass formation or extracavity iso- to hyper-echoic lesion, which may significantly interfere with the discrimination of local recurrences (Figs. 2A and B). The most common findings were persistent fluid collection in six patients (19.4%), including one case with organizing hematoma. Fat necrosis was found in four patients (12.9%). A benign-looking mass at the operation site was present in one other case (3.2%) (Table 2).
Oncoplastic surgery is defined as combination of excision of a tumor, with appropriate margin including lumpectomy or mastectomy, and immediate reconstruction of the breast.9 This technique has become rapidly more accepted throughout Western countries, enabling the achievement of oncologically safe margin and satisfactory cosmetic results.10 Recently, comparable results have been reported for local recurrence and survival outcome.11-13 Absorbable surgical mesh is the latest method in oncoplastic surgery. Since 2005, when the absorbable mesh was first introduced in Korea, a irranational survey showed that more than two third of breast surgeons had experience with the mesh insertion technique.5 The simplicity of the time-preserving technique that is easily applicable has been reported to have significant advantages for surgeons along with a relatively low cost and acceptable cosmetic outcomes.8 However, until now there has been insufficient evidence concerning the results of the mesh insertion. Previous studies were limited to the occasional report of observational analysis of immediate postoperative outcomes.
The majority of the clinicians agree that the mesh insertion involves the risk of wound complications or relatively prolonged use of antibiotics. In the national survey, the infection rate has been reported as up to 3.8%.5 On the contrary, another report with a longer follow-up period of 22 months after the operation showed a 14.3% higher complication rate and a relatively smaller number of patients.6 Our results revealed slightly higher incidence of wound complications compared to national survey. Up to now, infection has been the most commonly encountered problem, and the mesh is contraindicated in patients with possible wound problems or in higher risk patients such as diabetics or the elderly, though the specific age criteria has not been clearly stated.5 This study, in which the mean body mass index of 23.8 kg/m2, included no extraordinarily obese patients. Also, one patient was a tobacco user, but the patients did not suffer from an adverse outcome. Unfortunately, due to the small sample size of patients with complications, detailed analysis on development or preventive factors for complications were not available.
Although a statistical relationship was not established, adjuvant chemotherapy tended to affect the wound problem. Moreover, resolution of infectious complications required removal of the mesh. Some surgeons had recommended the use of prophylactic antibiotics, but until now, the requirement or the amount of necessary antibiotics has not been established. Also, radiation-induced skin reactions, such as erythema and hyperpigmentation, have been reported.8 After permanent implant insertion, the occurrence of complications such as infection, hematoma, and extrusion of the implant increase with more extensive history of irradiation.14 However, the relationship between radiation therapy and wound complication after absorbable mesh insertion is not clear.
Another major issue with mesh insertion in breast cancer patients is postoperative surveillance. Permanent insertion of a foreign body may induce inflammation or local response. Previous research on biomechanical materials using polyglactin 910, Vicryl® demonstrated a pronounced level of inflammation and an increased level of connective tissue formation at the interface.2 Histologically, perifilamentary inflammation occurs, resulting in chronic formation of foreign-body giant cells and lymphocytes in the periphery of the granuloma.2,15 Although the long-term changes have not yet been identified, the local response to the absorbable mesh may implicate future problems.
There have been several studies that suggested that the absorbable mesh insertion is not related to infectious complications. Góes, et al.16 reported that absorbable mesh insertion in the breast did not interfere with mammographic postoperative surveillance in detecting minute lesions such as calcifications and small nodules, but showed only minimal complications such as seroma and loss of areolar sensitivity, without causing wound infection. They reported that fat necrosis and cyst formation is common. However, breast cancer patients were not included in this analysis and the author emphasized that cancer patients are not a suitable subject because they require future radiation therapy and rigid control of tumor relapse.17 There were two Korean reports that investigated postoperative radiologic changes in the operation site after mesh insertion. According to these reports, the most common local finding was well-capsulated cyst formation with an iso-echoic, benign looking nodule.15,18 However, these studies are limited by a small number of patients and a rather short follow-up duration. There were no cases with infectious complications, but over-formation of fluid collection was found in one patient. Meanwhile, mammographic results or adjuvant treatments were not taken into account in the analysis.18
In this study, microcalcifications suggesting fat necrosis were found at the operation bed and the number of the microcalcifications was increased in the follow-up serial exam in three patients. Also, heterogeneous formations of mass densities were found in the mammographic surveillance in two patients. In the ultrasound exam, a mass-like shadow at the operation bed revealed various shapes of residual materials after absorption of the mesh. Similarly to previous reports, seven cases showed benign-looking granulation tissue; however, four patients were found to have a suspicious ill-defined mass that caused significant concern of local recurrence. Fat necrosis can be easily confused with breast lesions, which require differential diagnosis from malignancy in both clinical and radiological aspects. Fat necrosis may contain calcifications or fibrosis, which can appear as a speculated mass and may have a scirrhous feel upon examination. Potential fat necrosis or presentation of atypical calcifications should undergo core needle or excisional biopsy for pathologic confirmation.19-22
When planning treatment for breast cancer patients, long-term survival, local recurrence, psychological adjustment, functional competence, sexual adaptation and cosmetic outcome are all essential elements.3 A few studies had investigated the aesthetic outcome in mesh insertion patients and reported improved cosmetic satisfaction, psychological advantage, contour maintenance based on the questionnaire survey.6 77.6% of breast surgeons reported improved cosmetic outcomes after surgery, and 42.9% of surgeons reported higher patients satisfaction.5 On the other hand, 25% surgeon reported that the postoperative cosmetic results worsen as time passes.5 This study focuses mainly on the surgical and oncologic aspects of the postoperative outcome. From the viewpoint of cosmetically tolerable results with oncologic removal of breast cancer, reconstruction or oncoplastic surgery can be alternatives to mesh insertion. The inevitable adoption of invasive procedures for histologic confirmation for the vague lesion during follow up can generate a great deal of stress in breast cancer patients. If we consider that volume may decrease up to 47% after approximately one year,15 we should deliberate a carefully before deciding upon insertion of absorbable mesh for breast cancer patients, especially for those who are candidates for adjuvant chemo-radiation therapy.
In conclusion, absorbable mesh insertion has been reported to be a technically feasible and time-preserving procedure. However, follow-up results from an extended period have shown some significant considerations, such as interference in postoperative surveillance for local recurrences, chronic pain and wound infections. Therefore, we suggest that mesh insertion should be carefully considered and administered only in select cases. In postoperative radiologic surveillance, clinicians should be aware of the possible interference from mesh-related changes that may mimic local recurrence. Future investigation with long-term result is a prerequisite for clinical use of absorbable mesh for breast cancer patients.
Figures and Tables
Fig. 1
Mammographic findings after mesh insertion (A) various shapes of mass densities (arrow) were found, ranging from mild skin dimpling to heterogeneous mass densities. (B) Microcalcifications suggesting fat necrosis (circle) were found at the operation site.
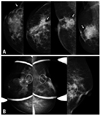
Fig. 2
Ultrasound findings after mesh insertion (A) Well-circumscribed cyst and ovoid shape nodules suggest granulomatous reaction to foreign body. (B) Ill-defined, irregular margined mass or extra-cavity lesions (arrows) may mimic local recurrence.

ACKNOWLEDGEMENTS
This work was supported by a grant of the Korea Healthcare technology R&D project, Ministry for Health & Welfare Affairs, Republic of Korea (A092255).
References
1. Sanuki J, Fukuma E, Wadamori K, Higa K, Sakamoto N, Tsunoda Y. Volume replacement with polyglycolic acid mesh for correcting breast deformity after endoscopic conservative surgery. Clin Breast Cancer. 2005. 6:175.

2. Klinge U, Schumpelick V, Klosterhalfen B. Functional assessment and tissue response of short- and long-term absorbable surgical meshes. Biomaterials. 2001. 22:1415–1424.

3. Morrow M, Strom EA, Bassett LW, Dershaw DD, Fowble B, Giuliano A, et al. Standard for breast conservation therapy in the management of invasive breast carcinoma. CA Cancer J Clin. 2002. 52:277–300.

4. Muss HB, Berry DA, Cirrincione CT, Theodoulou M, Mauer AM, Kornblith AB, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009. 360:2055–2065.

5. Kim KS, Park MY, Kim WJ, Na KY, Jung YS, Choi YJ, et al. Nationwide Survey of the Use of Absorbable Mesh in Breast Surgery in Korea. J Breast Cancer. 2009. 12:210–214.

6. Lee JH, Hong YI, Jeong JH, Lee JI, Lee JH, Moon HJ, et al. Volume replacement with polyglactin 910 mesh for breast reconstruction after endoscopy-assisted breast conserving surgery for treating early breast cancer: the early results. J Breast Cancer. 2009. 12:193–198.

7. Eom TI, Kim BS, Koo BY, Kim JW, Lim YA, Lee HH, et al. The Use of a Corrective Procedure with Vicryl Mesh for Oncoplastic Surgery of the Breast. J Breast Cancer. 2009. 12:36–40.

8. Kim HO, Hwang SI, Yom CK, Park YL, Bae WG. The use of absorbable surgical mesh after partial mastectomy for improving the cosmetic outcome. J Breast Cancer. 2009. 12:151–155.

9. Audretsch W, Rezai M, Kolotas C, Zamboglou N, Schnabel T, Bojar H. Tumor-Specific Immediate Reconstruction in Breast Cancer Patients. Perspect Plast Surg. 1998. 11:71–100.

10. Clough KB, Kaufman GJ, Nos C, Buccimazza I, Sarfati IM. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol. 2010. 17:1375–1391.

11. Fitoussi AD, Berry MG, Famà F, Falcou MC, Curnier A, Couturaud B, et al. Oncoplastic breast surgery for cancer: analysis of 540 consecutive cases [outcomes article]. Plast Reconstr Surg. 2010. 125:454–462.

12. Caruso F, Catanuto G, De Meo L, Ferrara M, Gallodoro A, Petrolito E, et al. Outcomes of bilateral mammoplasty for early stage breast cancer. Eur J Surg Oncol. 2008. 34:1143–1147.

13. Rietjens M, Urban CA, Rey PC, Mazzarol G, Maisonneuve P, Garusi C, et al. Long-term oncological results of breast conservative treatment with oncoplastic surgery. Breast. 2007. 16:387–395.

14. Cordeiro PG. Breast reconstruction after surgery for breast cancer. N Engl J Med. 2008. 359:1590–1601.

15. Kwag HJ. Imaging findings of implanted absorbable mesh in patients with breast partial resection. Yonsei Med J. 2008. 49:111–118.

16. Góes JC, Landecker A, Lyra EC, Henríquez LJ, Góes RS, Godoy PM. The application of mesh support in periareolar breast surgery: clinical and mammographic evaluation. Aesthetic Plast Surg. 2004. 28:268–274.

17. Góes JC. Periareolar mammaplasty: double skin technique with application of polyglactine or mixed mesh. Plast Reconstr Surg. 1996. 97:959–968.

18. Choi Y, Hong HP, Kwag HJ. Ultrasonographic findings of an implanted absorbable mesh in patients with breast partial resection: a preliminary study. J Korean Soc Ultrasound Med. 2007. 26:89–94.
19. Harris JR, Lippman ME, Morrow M, Osborne CK. Disease of the breast. 2009. 4th ed. Philadelphia: Wolters Kluwer.
20. Symmers W. Systemic pathology. 1978. Edinburgh: Churchill Livingstone.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download