Abstract
Purpose
Combined hepatocellular-cholangiocarcinoma (CHCC) is an uncommon form of cancer, and its clinicopathological features have rarely been reported in detail. This study was undertaken to evaluate the clinicopathological characteristics and prognostic factors of CHCC.
Materials and Methods
The clinicopathological features of patients diagnosed with CHCC at Severance Hospital between January 1996 and December 2007 were retrospectively studied by comparing them with the features of patients with hepatocellular carcinoma (HCC) or cholangiocarcinoma (CC) who had undergone a hepatic resection during the same period.
Results
Forty-three patients diagnosed with CHCC were included in this study (M : F=35 : 8, median age, 55 years). According to the parameters of the American Joint Committee on Cancer staging, there were 6 (14.0%), 9 (20.9%), 25 (58.1%), and 3 (7.0%) patients with stages I, II, III, and IV cancer, respectively. Thirty-two of the 43 patients underwent resection with curative intent. After resection, 27 patients (84.4%) had tumor recurrence during the follow-up period of 18 months (range: 6-106 months), and the median time to recurrence was 13 months. Overall median survival periods after hepatic resection of CHCC, HCC and CC were 34, 103 and 38.9 months, respectively (p<0.001). The median overall survival for all patients with CHCC was 21 months, and the 5-year survival rate was 18.1%. The presence of portal vein thrombosis and distant metastasis were independent prognostic factors of poor survival.
Primary liver cancer is a major worldwide health problem. Most primary cancers of the liver are classified into two major types: hepatocellular carcinoma (HCC), which originates in the hepatocytes, and cholangiocarcinoma (CC), which originates from the epithelial cells in the bile duct. However, a small proportion of tumors, namely combined hepatocellular-cholangiocarcinoma (CHCC), show a mixture of hepatocellular and glandular features.1-3 According to the World Health Organization classification, this condition, defined as a tumor containing components of both HCC and CC,4 is a very rare form of primary liver cancer in which dual differentiation toward hepatocytes and bile duct epithelia coexist.5 Since Allen and Lisa first described the features of this tumor in 1949,6 few reports have examined its clinical features, survival outcomes, or prognostic factors. Many reports have increasingly raised concerns regarding CHCC. Because of its low prevalence, however, little is known about the clinicopathological characteristics and prognoses of patients with CHCC, and comparing the outcome of patients with CHCC to that of patients with HCC or CC has yielded conflicting results in a few studies. Some investigators have suggested that the biological features of CHCC resemble those of HCC rather than those of CC,7 and that the prognosis for patients with CHCC is worse than for pure HCC and better than for pure CC.1,2 In contrast, Jarnagin, et al.8 reported that the clinical features of CHCC were more similar to those of CC, and that the 5-year survival rate of patients with CHCC was lower than that of patients with either HCC or CC. To fully clarify the characteristics, survival, and prognostic factors of CHCC, we retrospectively analyzed the clinicopathological characteristics and prognostic factors related to survival and recurrence in patients diagnosed with CHCC at a single center. We also compared patients with CHCC to those with HCC or CC who had undergone an operation during the same study period.
We studied 43 patients who were diagnosed with CHCC, as confirmed by pathologic findings, at Severance Hospital between January 1996 and December 2007. Pathological specimens were obtained via needle biopsy in 11 patients and hepatectomy in 32. The median follow-up period was 18 months (range: 6-106 months). The data records of 368 patients with ordinary HCC and 128 patients with CC who had undergone an operation during the same period were selected and reviewed for comparison. This study was approved by the local institutional review board.
A retrospective review of a database provided information about all the patients. The parameters reviewed included gender, age, clinical presentation, alcohol consumption, viral hepatitis B and C status, patterns of contrast-enhanced dynamic CT (HCC-like pattern, CC-like pattern, and mixed pattern), laboratory data [serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), total bilirubin, albumin, and prothrombin time (PT)]. Each CT was read by two radiologists who had no information about the pathological results. An HCC-like pattern was defined as high attenuation/signal intensity in the early phase and low attenuation/signal intensity in the delayed phase. A CC-like pattern was defined as low attenuation/signal intensity in the early phase, and iso- or high attenuation/signal intensity in the delayed phase. A mixed pattern was defined as a mix of both of these patterns.9,10 To evaluate the degree of hepatic disease, the Child-Pugh status was defined in each patient.
In all of the selected patients, we obtained histopathologic information regarding the size, number, and location of tumor(s), presence of capsule, portal vein thrombosis, lymph node metastasis, intrahepatic metastasis, and distant metastasis. Tumor staging was performed in accordance with the American Joint Committee on Cancer (AJCC) staging system, 6th edition. The surgical method was categorized as limited resection, in which the segment containing the tumor was resected; a major hepatic resection, in which more than a single segment was resected; and liver transplantation.
With respect to survival outcomes, we analyzed median survival time, 1-year, 3-year, and 5-year survival rates, median disease-free survival, cumulative recurrence rates, and recurrence sites. The prognostic factors analyzed were age, sex, presence of cirrhosis, hepatic function (Child-Pugh class), positive serum viral marker, tumor size, number of tumors, patterns of CT, presence of capsule, portal vein thrombosis, lymph node metastasis, distant metastasis, and AJCC stage.
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS version 16.0). Data values are reported as the median (range) or as n (%). Student's t-tests and χ2-tests were used for statistical analysis. Univariate analysis was performed to identify predictors of survival using the Kaplan-Meier method, and survival curves were compared using the log-rank test. The results of the univariate analysis were considered significant if the probability of occurrence by chance was 5% or less (p<0.05). For continuous variables, the cutoff was set at the median value. Multivariate analysis was performed using Cox proportional hazards regression analysis.
The baseline characteristics of 43 patients with CHCC are provided in Table 1. The median age was 55 years (range: 30-72 years); there were 35 men (81.4%) and eight women (18.6%). The prevalence of the hepatitis B surface antigen was 62.8%, and positive serology for hepatitis C infection was found in 14.0% of patients. Underlying liver cirrhosis was found in 29 (67.4%) patients. If we assumed that all the patients had liver cirrhosis, 31 (72.1%) were classified as Child-Pugh class A, and 12 (27.9%) were in Child-Pugh class B. Regarding patterns of contrast-enhanced dynamic CT, 28 patients exhibited an HCC-like pattern, 12 exhibited a CC-like pattern, and three exhibited a mixed pattern.
According to the AJCC staging classification, 6 patients (14.0%) were in stage I, 9 (20.9%) in stage II, 25 (58.1%) in stage III, and three (7.0%) in stage IV. Thirty-two patients (74.4%) were pathologically diagnosed by operation and 11 patients (25.6%) by gun-biopsy. Nine patients (21.0%) underwent medical treatment; systemic chemotherapy (one patient), intra-arterial chemotherapy (four patients), and transarterial chemo-embolization (four patients). One patient underwent radiotherapy, and another refused treatment. Among five patients who had treated systemic or intra-arterial chemotherapy, most of them (four) had received a platinum complex-based regimen (systemic/intra-arterial cisplatin or intra-arterial carboplatin). One patient received combination chemotherapy with systemic 5-fluorouacil and cisplatin. The other received combination chemotherapy of systemic adriamycin and mitomycin.
Thirty-two patients (74.4%) died during the follow-up period, and the median overall survival was 21 months (range: 6-106 months). The 1-year overall survival rate was 69.0%, the 3-year overall survival rate 34.1%, and the 5-year overall survival rate was 18.1% (Fig. 1).
Univariate analysis identified the predictive factors of overall survival as absence of cirrhosis, negative serum viral marker, presence of capsule formation, portal vein thrombosis, distant metastasis and advanced AJCC stage. In the stepwise Cox multivariate regression analysis, the presence of portal vein thrombosis and distant metastasis had an independent effect on overall survival (Table 2). The multivariate relative risk for the presence as compared to the absence of portal vein thrombosis was 2.745 [95% confidence interval (CI), 1.192-6.232, p=0.018], and the risk for the presence of distant metastasis was 21.685 (95% CI, 3.276-143.555, p=0.001).
Twenty-seven of 32 patients who had undergone curative resection (84.4%) had recurrence during the follow-up period, and the median disease-free survival was 13 months (range, 2-41 months). Among 27 patients with recurrent CHCC, intrahepatic metastases were detected in 13 patients (40.6%), and extrahepatic metastases were detected in 14 (43.8%). After recurrence, 27 patients underwent further treatment, including reoperation (eight patients), systemic chemotherapy (seven patients), trans-arterial chemo-embolization (three patients), intra-arterial chemotherapy (one patient), and radiotherapy (one patient). Seven patients had only supportive care after detection of recurrence. In the univariate analysis, predictive factors of recurrence were the presence of lymph node metastasis and distant metastasis (p=0.016 and <0.001, respectively). Although the presence of distant metastasis was still significant [relative risk 21.685 (95% CI, 2.698-67.394)] in the multivariate analysis as expected, the relative risk of the presence of lymph node metastasis was not significant (p=0.690) (data not shown).
To compare outcomes after hepatic resection, 32 patients who had been diagnosed by surgical operation were compared with 368 HCC patients and 128 CC patients who had been diagnosed by pre-operative evaluation studies (imaging modalities and tumor markers) and had undergone an operation during the same period. The clinical features of the three patient groups are summarized in Table 3. Significant differences were observed in the status of viral infection, the presence of cirrhosis, Child-Pugh status, AST, ALT, and albumin level of the three groups. Positive serum HBs Ag was most frequently showed in patients with HCC (81.2% vs. 68.8%, 7.8%, p<0.001), and positive anti-HCV Ab was relatively more frequent in patients with CHCC (15.6% vs. 6.8%, 1.6%, p=0.007). The prevalence of cirrhosis in patients with CHCC (75.0%) was high compared to patients with HCC (53.5%) and CC (15.6%) (p=0.025). Child-Pugh class A was more frequently found in the HCC group than in the CHCC and CC groups (p=0.006). Levels of AST and ALT were significantly higher in the CHCC group (p=0.047, 0.037, respectively), and the level of albumin in the CHCC group was lower than in the other two groups (p=0.029). Even though the difference was not significant, the total bilirubin levels were slightly higher in the CHCC group than in the other two groups (p=0.070). All these results show that patients with CHCC had badly preserved liver functions at the time of operation.
Surgical methods and pathological characteristics for the three groups are provided in Table 4. Tumor size and location were similar between the three groups (p=0.085, p=0.053, respectively). Differences in the number of tumors, the presence of capsules, portal vein thrombosis, and the frequency of advanced staged tumors were observed between the three groups. Multifocal disease was seen more frequently in the CHCC group than in the other two groups (p<0.001). The CHCC group had intermediate prevalence of capsule formation and portal vein thrombosis compared to the HCC group and CC group (43.8% vs. 75.4% vs. 25.0%, 37.5% vs. 5.8% vs. 57.8%, p<0.001 for both). Lymph node metastasis in CHCC occurred with a similar frequency as in CC (12.5% vs. 26.6%, p=0.095), and significantly more frequently than in HCC (12.5% vs. 6.8%, p<0.001). The frequency of intrahepatic metastasis and the proportion of advanced tumor stage in the CHCC group were higher than in the HCC group and the CC group (p=0.015, 0.036, respectively).
After resection for combined tumors, the one-year survival rate was 79.7%; the three-year survival rate 45.0%; and the five-year survival rate was 23.8%. All of these were lower than those of the HCC group (91.9%, 74.8%, and 69.1%, respectively), and comparable with those of the CC group (76.6%, 52.7% and 39.5%, respectively) (Fig. 2). The median survival time for the CHCC group (34 months) was significantly shorter than for the HCC and CC groups (103 and 38.9 months, respectively; p<0.001).
Twenty-seven patients (84.4%) in the CHCC group had a recurrence during the follow-up period, which was a recurrence rate much higher than that of HCC (52.7%) or CC (64.1%) (p<0.001). The median disease-free survival was 13 months with CHCC, 10 months with HCC group and 12.3 months with CC, and these differences were not statistically significant (p=0.562).
Since the first comprehensive description about primary liver cancer comprising elements of HCC and CC was published in 1949,6 several studies have been performed regarding the clinicopathological features of CHCC. Because CHCC is encountered infrequently, useful clinical data is limited. Moreover, only a few reports give information about surgical treatment and clinical outcomes after hepatic resection.
The incidence of CHCC varies considerably, from 2.4% reported by Goodman, et al.11 to 14.2% from the data series of Allen and Lisa.6 Liu, et al.12 reported an incidence of 2.0%, and the Liver Cancer Study Group of Japan showed that the CHCC group accounted for 1.2% of surgical cases and 1.6% of autopsy cases.7 To estimate the incidence in our study, we included only patients who had liver cancer and underwent hepatic resection, because CHCC could be missed or misdiagnosed as HCC in patients who underwent biopsy only. Thus, among the 972 patients who underwent hepatic resection for primary liver cancer in our institution, 32 were found to have CHCC, an incidence rate of 3.3%.
The cellular origin of CHCC cells remains unclear. Three possibilities have been proposed for their histogenesis: 1) the cells represent double cancer with dual differentiation of hepatocytes and bile duct epithelium; 2) the cancer initially developed from either hepatocytes or bile duct epithelium and differentiated from other components; or 3) the cancer developed in intermediate cells that subsequently differentiated into CHCC.12
Histologic diagnosis of CHCC depends on the demonstration of dual differentiation of hepatocellular and biliary epithelial features, with distinct immunohistochemical features demonstrating malignant transformation in both hepatic and biliary cells.13,14 Radiologically, Lin G, et al.15 reported that the imaging pattern for CHCC can be an HCC-like pattern, a CC-like pattern, or a mixed pattern. On the other hand, some authors have suggested that a target-like appearance on the sonography, presented as a hypovascular mass with central hypervascular portions on angiography, revealed an enhancing pattern around or within the mass on the enhanced CT that may be helpful in making an accurate radiologic diagnosis of CHCC.16,17 However, the diagnostic criteria for CHCC remain controversial because clinical experience with patients with CHCC from a single center is very limited. Moreover, the clinical characteristics of CHCC are inconsistent due to discrepancies in the diagnostic criteria and clinical populations in published studies. In our studies, most of the CHCC patients (65.1%) showed an HCC-like pattern in the pre-operative enhanced CT. Without histological evidence, these patients may have been misdiagnosed with HCC. Therefore, it is very difficult for patients with CHCC to be correctly diagnosed before undergoing an operation. Because diagnosis is difficult during the pre-operative period, the true incidence is likely to be much higher than what has been reported, our series included. Because CHCC has characteristics of both portal vein invasion of HCC and lymph node metastasis of CC, patients with CHCC were in a more advanced stage of the disease than were those with HCC or CC. Because the most effective treatment of CHCC is surgical intervention, early diagnosis with developed radiological studies and valuable clinical parameters is very important.
In previous studies of biological behavior and clinicopathological features of CHCC, CHCC was not regarded simply as a combination of ordinary HCC and ordinary CC. Jarnagin, et al.8 reported that CHCC more closely resembled CC and that the biliary differentiation component of CHCC may contribute to poor prognosis. Ng, et al.13 reported that the clinicopathological features of CHCC were similar to those of HCC. Other studies8,12 have shown varied prevalence of viral hepatitis and underlying cirrhotic liver in the CHCC group compared to those in the HCC or CC group. In our study, the prevalence of underlying cirrhosis in the liver was higher in patients with CHCC than in the HCC and CC groups, which indicates that patients with CHCC had less well-preserved liver function at the time of operation.
When comparing the characteristics of CHCC to HCC and CC, S. Chantajitr, et al.18 and Jarnagin, et al.8 found no differences in tumor size, the number of tumors, the presence of major vascular invasion (portal vein and hepatic vein), or the presence of lymph node metastasis between the three groups. In contrast, our study showed higher frequencies of multifocal tumors and portal vein thrombosis, similar to data reported by Koh, et al.1 and Yano, et al.14 In addition, an advanced AJCC stage and the presence of intrahepatic metastasis were more frequently found among CHCC patients. Lymph node metastasis of CHCC was comparable with that of CC, and significantly higher than that of HCC.
Few have reported on the prognosis of CHCC after surgical resection. Most studies have shown that survival of patients with CHCC was poorer than that of HCC patients.1,3,8,14,19 In contrast, the comparison of prognosis between CHCC and CC is still controversial.1,2,14 Our study demonstrated that patients with CHCC tended to have poorer survival outcomes than those with either HCC or CC. Okuda20 reported that patient prognosis with CHCC was poorer than for HCC because lymph node metastasis occurs more frequently. For this reason, our results showing more frequent lymph node metastasis in CHCC than in HCC might explain the poorer survival outcomes for CHCC than for HCC. In addition, several characteristics of CHCC ascertained above might contribute to poor survival outcome: poorly preserved liver function, more frequent multiplicity and more frequent intrahepatic metastasis
Meanwhile, in the comparison of survival outcomes based on tumor stages, no significant differences were observed between the two groups, especially in the earlier tumor stages. This result suggests that a poor survival outcome of CHCC might be attributed mainly to more advanced tumor status at the time of surgery. Vulnerability to multiplicity, intrahepatic metastasis and lymph node metastasis of CHCC would be reasons for more advanced tumor status, and these findings collectively suggested unfavorable tumor biology of CHCC.7 This unfavorable behavior of CHCC may be connected with tumor cells originating from pleuripotent hepatic precursor cells.21,22 Therefore, further study will be necessary to elucidate the features of tumor cells in CHCC. Also, based on the comprehension of characteristic behaviors of CHCC, a proper preoperative work-up and early diagnosis of CHCC would certainly be important to improve its survival rate.
We recognize several limitations in our study. This study was retrospective and could have been affected by any and all of the limitations of this investigational design. The possibility of selection bias is another potential limitation of this study. Moreover, the comparison of the CHCC, HCC and CC groups with different tumor characteristics and stages had an inherent bias that made our conclusions of poor value regarding tumor recurrence and survival rates. This was because many cases that were considered to be operable were later found in the operating room to be more progressed than they had appeared in the pre-operative studies. Therefore, precise preoperative evaluation will be necessary to improve clinical outcomes for patients with CHCC. Like most other reports on CHCC, our study had a small number of patients, because the data were taken from a single center, which might have hampered the identification of possible important predictive factors. Because the incidence of CHCC is low, multicenter studies would be required to investigate postoperative adjuvant therapy and multimodality treatment for CHCC.
In conclusion, most reports have indicated that surgical intervention, including resection of lymph nodes, was the only effective treatment for CHCC. However, patients with CHCC have a significantly poorer survival outcome after hepatic resection than do patients with HCC or CC. The presence of portal vein thrombosis appears to be useful in predicting the prognosis of patients with CHCC, and further studies on effective treatment modalities and clinical predictors for CHCC are required to prolong the survival of these patients.
Figures and Tables
 | Fig. 1Kaplan-Meier overall survival rate of combined hepatocellular-cholangiocarcinoma (CHCC) (n=43). |
 | Fig. 2Kaplan-Meier overall survival rate of combined hepatocellular-cholangiocarcinoma (CHCC) (n=32), HCC (n=368) and CC (n=128). HCC, hepatocellular-carcinoma; CC, cholangiocarcinoma. |
Table 1
Baseline Characteristics of Patients with CHCC (n=43)
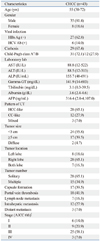
CHCC, combined hepatocellular-cholangiocarcinoma; HBsAg, hepatitis B virus surface antigen; HCV Ab, hepatitis C virus antibody; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; Gamma-GT, gamma-glutamyl transferase; AFP, alpha-fetoprotein; CC, cholangiocarcinoma.
Results are expressed as median (range) or n (%).
*Child-Pugh A includes non-cirrhotic hepatitis.
†AJCC 6th, American Joint Committee on Cancer staging system, 6th edition.
ACKNOWLEDGEMENTS
This study was supported by grant from the Good Health R & D Project of the Ministry of Health and Welfare, Republic of Korea (A050021).
References
1. Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, et al. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005. 189:120–125.

2. Maeda T, Adachi E, Kajiyama K, Sugimachi K, Tsuneyoshi M. Combined hepatocellular and cholangiocarcinoma: proposed criteria according to cytokeratin expression and analysis of clinicopathologic features. Hum Pathol. 1995. 26:956–964.

3. Taguchi J, Nakashima O, Tanaka M, Hisaka T, Takazawa T, Kojiro M. A clinicopathological study on combined hepatocellular and cholangiocarcinoma. J Gastroenterol Hepatol. 1996. 11:758–764.

4. Gibson JB, Sobin LH. Histological typing of tumours of the liver, biliary tract, and pancreas. 1978. Geneva: World Health Organization.
5. Ishak KG, Anthony PP, Sobin LH. Histological typing of tumors of the liver. 1994. 2nd ed. Berlin: Springer.
6. ALLEN RA, LISA JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949. 25:647–655.
7. Choi SB, Kim KS, Choi JY, Park SW, Choi JS, Lee WJ, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009. 16:3048–3056.

8. Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002. 94:2040–2046.
9. Honda H, Onitsuka H, Yasumori K, Hayashi T, Ochiai K, Gibo M, et al. Intrahepatic peripheral cholangiocarcinoma: two-phased dynamic incremental CT and pathologic correlation. J Comput Assist Tomogr. 1993. 17:397–402.
10. Honda H, Ochiai K, Adachi E, Yasumori K, Hayashi T, Kawashima A, et al. Hepatocellular carcinoma: correlation of CT, angiographic, and histopathologic findings. Radiology. 1993. 189:857–862.

11. Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985. 55:124–135.

12. Liu CL, Fan ST, Lo CM, Ng IO, Lam CM, Poon RT, et al. Hepatic resection for combined hepatocellular and cholangiocarcinoma. Arch Surg. 2003. 138:86–90.

13. Ng IO, Shek TW, Nicholls J, Ma LT. Combined hepatocellular-cholangiocarcinoma: a clinicopathological study. J Gastroenterol Hepatol. 1998. 13:34–40.

14. Yano Y, Yamamoto J, Kosuge T, Sakamoto Y, Yamasaki S, Shimada K, et al. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol. 2003. 33:283–287.

15. Lin G, Toh CH, Wu RC, Ko SF, Ng SH, Chou WC, et al. Combined hepatocellular cholangiocarcinoma: prognostic factors investigated by computed tomography/magnetic resonance imaging. Int J Clin Pract. 2008. 62:1199–1205.

16. Bhagat V, Javle M, Yu J, Agrawal A, Gibbs JF, Kuvshinoff B, et al. Combined hepatocholangiocarcinoma: case-series and review of literature. Int J Gastrointest Cancer. 2006. 37:27–34.

17. Kim H, Park MS, Park YN, Kim H, Kim KS, Choi JS, et al. Preoperative radiologic and postoperative pathologic risk factors for early intra-hepatic recurrence in hepatocellular carcinoma patients who underwent curative resection. Yonsei Med J. 2009. 50:789–795.

18. Chantajitr S, Wilasrusmee C, Lertsitichai P, Phromsopha N. Combined hepatocellular and cholangiocarcinoma: clinical features and prognostic study in a Thai population. J Hepatobiliary Pancreat Surg. 2006. 13:537–542.

19. Lee WS, Lee KW, Heo JS, Kim SJ, Choi SH, Kim YI, et al. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today. 2006. 36:892–897.

20. Okuda K. Natural history of hepatocellular carcinoma including fibrolamellar and hepato-cholangiocarcinoma variants. J Gastroenterol Hepatol. 2002. 17:401–405.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download