Abstract
Erlotinib is accepted as a standard second-line chemotherapeutic agent in patients with non-small cell lung cancer who are refractory or resistant to first-line platinum-based chemotherapy. There has been no previous report of bowel perforation with or without gastrointestinal metastases related to erlotinib in patients with non-small cell lung cancer. The exact mechanism of bowel perforation in patients who received erlotinib remains unclear. In this report, we report the first case of enterocutaneous fistula in a female patient with metastatic non-small cell lung cancer 9 months, following medication with erlotinib as second-line chemotherapy.
Chemotherapy is a mainstay treatment for advanced or metastatic non-small cell lung cancer (NSCLC).1 Erlotinib, an epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI), has been shown to be effective for Asian patients with favorable clinical features, such as female gender, never or non-smoker, and adenocarcinoma.2 The most common adverse effect is skin rash. However, other adverse effects are relatively minor and manageable.2-6
Among several targeted agents with different mechanisms of action, patients treated with vascular endothelial growth factor (VEGF)-targeted agents, such as bevacizumab, develop hypertension, thrombosis, gastrointestinal bleeding, and bowel perforation as uncommon adverse effects.7-11 However, there has been no previous report of gastrointestinal perforation or fistula related to erlotinib administration.
Here, we report a case of enterocutaneous fistula in a patient with NSCLC after the ninth course of erlotinib treatment as a second-line palliative chemotherapy.
A 66-year-old female was diagnosed with metastatic non-small cell adenocarcinoma of the lung [NSCLC; cT4N0M1 for metastases of the lung to the contralateral lung and pleura on 18F-fluorodeoxyglucose positron emission tomography computed tomography (FDG-PET CT) scan] in January 2008. The patient had a history of exploratory laparotomy and a total hysterectomy, due to acute abdomen about 30 years earlier. Physical examination revealed sigmoid colon herniation through an abdominal wall defect, with no sign or symptom of strangulation. Although surgical management of the herniated colon loop and closure of the abdominal wall were strongly recommended, she refused further surgical treatment, because there had been no symptom, such as abdominal pain or bowel habit abnormality associated with the sigmoid colon herniation, over the previous 30 years.
She was treated with a first-line chemotherapy, including cisplatin (70 mg/m2, day 1) and docetaxel (70 mg/m2, day 1) every 3 weeks for 6 cycles from January 2008 to July 2008. Following completion of 6 cycles of first-line chemotherapy, a chest CT scan showed a partial response, according to the Response Evaluation Criteria in Solid Tumors (RECIST).
In October 2008, second-line chemotherapy with erlotinib was started because a follow-up chest CT scan revealed regrowth of the primary lung mass in the left upper lobe and increased malignant pleural effusion. A subsequent chest CT scan showed persistent partial response at the first, third, fifth, and seventh month following erlotinib administration.
In November 2008 (seventh month following erlotinib administration), the patient complained of low back pain after a fall at home, and a compression fracture of the fifth lumbar vertebra was detected on a simple spine X-ray and spine magnetic resonance image (MRI). Percutaneous vertebroplasty and concomitant bone biopsy were performed at the Department of Neurosurgery. However, no evidence of bone metastasis was detected in the bone biopsy specimen, which was compatible with a benign compression fracture related to severe osteoporosis. Additionally, ibuprofen (200 mg three times daily) was added to relieve low back pain for 7 days after the procedures.
Nine months after starting second-line chemotherapy with erlotinib, the patient was admitted with purulent yellowish sputum, cough, and mild shortness of breath without abdominal symptoms. Chest CT scan demonstrated decreased size of the primary lung mass, which was still evaluated as a partial response, with focal, patchy consolidation and peribronchial thickening in the left lung field, clinically compatible with pneumonia.
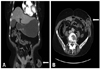
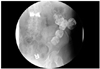
On physical examination, a coarse breathing sound with a crackle, especially in the left lung field, was detected, and an enterocutaneous fistula with leakage of fecal material was found in the previous sigmoid colon herniation through the abdominal wall defect (Fig. 1A and B). Nevertheless, no sign of strangulation, including tenderness or rebound tenderness, was observed. Fistulography also demonstrated passage of contrast media into the sigmoid colon without another fistula (Fig. 2). Further administration of erlotinib was withheld after documentation of the enterocutaneous fistula.
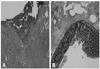
After resolution of pneumonia via empirical antibiotic treatment for 14 days, a scheduled sigmoid colon resection and primary anastomosis were performed at the Department of General Surgery. Surgical pathologic findings included submucosal edema and serosal hemorrhage of the perforated sigmoid colon, consistent with enterocutaneous fistula and no metastatic tumor foci in the sigmoid colon (Fig. 3A and B). Ten days postoperatively, the patient was discharged with complete recovery from the enterocutaneous fistula. She was alive and well on regular follow-up at the outpatient clinic, with no evidence of lung cancer progression.
To our knowledge, there is no previously reported case of enterocutaneous fistula without intestinal metastasis in a patient with NSCLC who received erlotinib.
Hoffman-La Roche Ltd. announced that gastrointestinal perforation (including fatalities) has been reported in patients receiving erlotinib, and the prescribing information indicates that patients receiving concomitant anti-angiogenic agents, corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and/or taxane-based chemotherapy, or who have a prior history of peptic ulceration or diverticular disease, are at increased risk of gastrointestinal perforation.12
Our patient had risk factors related to gastrointestinal perforation during medication with erlotinib. First, she had a history of previous abdominal surgery, which was eventually complicated with sigmoid colon herniation through an abdominal wall defect. This condition may cause a continuous increase of the pressure on already weakened areas of the bowel, thus further predisposing patients to bowel perforations or fistulae that re-epithelialize poorly during erlotinib administration.13,14 Second, NSAIDs were administered concomitantly for 7 days following percutaneous vertebroplasty, which could block COX 1 and COX 2 enzyme activity, also leading to gastrointestinal perforations through the development of ulcers and poor ulcer healing.13,14 There is strong evidence that HER-1/EGFR and VEGF share a common downstream signaling pathway both in vitro and in vivo, and they exert effects on tumor cells directly or indirectly.15-18
Although we suggest that potent cytotoxic agents can lead to bowel perforation regardless of their mechanism of action, especially in patients with visceral metastases caused by tumor regression and necrosis, our patient showed no evidence of bowel metastasis, according to radiologic findings at initial diagnosis or during administration of erlotinib, or pathologic findings of the post-operative resected bowel specimen.
In conclusion, physicians should be aware of the rare, but possible, adverse effect of bowel perforation or fistula following administration of erlotinib, even in NSCLC patients without visceral metastases, and erlotinib administration should be discontinued when patients develop gastrointestinal perforation.
Figures and Tables
Fig. 1
(A and B) White arrow indicates an enterocutaneous fistula in the sigmoid colon herniation through the abdominal wall defect.
References
1. Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2010. CD007309.
2. Uhm JE, Park BB, Ahn MJ, Lee J, Ahn JS, Kim SW, et al. Erlotinib monotherapy for stage IIIB/IV non-small cell lung cancer: a multicenter trial by the Korean Cancer Study Group. J Thorac Oncol. 2009. 4:1136–1143.

3. Dudek AZ, Kmak KL, Koopmeiners J, Keshtgarpour M. Skin rash and bronchoalveolar histology correlates with clinical benefit in patients treated with gefitinib as a therapy for previously treated advanced or metastatic non-small cell lung cancer. Lung Cancer. 2006. 51:89–96.

4. Mohamed MK, Ramalingam S, Lin Y, Gooding W, Belani CP. Skin rash and good performance status predict improved survival with gefitinib in patients with advanced non-small cell lung cancer. Ann Oncol. 2005. 16:780–785.

5. Hotta K, Kiura K, Ueoka H, Tabata M, Fujiwara K, Kozuki T, et al. Effect of gefitinib ('Iressa', ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer. 2004. 46:255–261.

6. Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003. 290:2149–2158.

7. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009. 27:1227–1234.

8. Di Costanzo F, Mazzoni F, Micol Mela M, Antonuzzo L, Checcacci D, Saggese M, et al. Bevacizumab in non-small cell lung cancer. Drugs. 2008. 68:737–746.

9. Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004. 22:2184–2191.

10. Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist. 2007. 12:356–361.

11. Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol. 2007. 14:1860–1869.

12. Johnson JR, Cohen M, Sridhara R, Chen YF, Williams GM, Duan J, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res. 2005. 11:6414–6421.

13. Schellhaas E, Loddenkemper C, Schmittel A, Buhr HJ, Pohlen U. Bowel perforation in non-small cell lung cancer after bevacizumab therapy. Invest New Drugs. 2009. 27:184–187.

14. Badgwell BD, Camp ER, Feig B, Wolff RA, Eng C, Ellis LM, et al. Management of bevacizumab-associated bowel perforation: a case series and review of the literature. Ann Oncol. 2008. 19:577–582.

15. Kim SJ, Uehara H, Karashima T, Shepherd DL, Killion JJ, Fidler IJ. Blockade of epidermal growth factor receptor signaling in tumor cells and tumor-associated endothelial cells for therapy of androgen-independent human prostate cancer growing in the bone of nude mice. Clin Cancer Res. 2003. 9:1200–1210.
16. de Jong JS, van Diest PJ, van der Valk P, Baak JP. Expression of growth factors, growth-inhibiting factors, and their receptors in invasive breast cancer. II: Correlations with proliferation and angiogenesis. J Pathol. 1998. 184:53–57.

17. Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997. 151:1523–1530.
18. Bruns CJ, Harbison MT, Davis DW, Portera CA, Tsan R, McConkey DJ, et al. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res. 2000. 6:1936–1948.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download