Abstract
We report herein a case of hyperacute onset of spontaneous cervical spinal subdural hematoma treated with methylprednisolone pulse therapy that showed good results. A 57-year-old man was admitted for posterior neck pain and paraparesis which occurred an hour ago. MRI revealed a ventral subdural hematoma distributed from the level of C1 down to T3, compressing the spinal cord. Conservative management with methylprednisolone pulse therapy was administered considering the patient's poor general condition. Although emergent surgical decompression is necessary in most cases of spinal subdural hematoma, conservative management with steroid therapy could be effective.
Spinal subdural hematoma (SSH) may be caused by major or minor trauma or lumbar puncture.1 Spontaneous SSH often develops in patients with coagulation defects or on anticoagulants therapy. SSH rarely involves the cervical region, and to our knowledge few such cases have been previously reported in the literature.2-4
Preferable treatment of SSH is surgical decompressive laminectomy and drainage of hemorrhage,5 however, conservative care also resulted in spontaneous resolution of hematoma in some cases.4 We report a case of hyperacute onset of spontaneous cervical SSH in a patient with no coagulopathy and its treatment with methylprednisolone pulse therapy which produced good results.
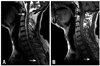
A 57-year-old man was admitted to the hospital with sudden and severe posterior neck and shoulder pains extending to the suboccipital region that developed paraparesis one hour ago. He denied any antecedent trauma, exertional activity, sneezing, valsalva maneuver, coitus, or vomiting. The patient had been on hypertension medication for 10 years and was diagnosed with chronic renal failure seven years ago. He had received hemodialysis three times per week for the past five years. The patient's last hemodialysis was performed two days before the admission. He had no previous history of bleeding tendency including taking antiplatelet or anticoagulant agents. At admission, blood pressure was 160/100 mmHg and pulse rate was normal range. Neurological examination of the cranial nerve function showed normal results. However, the patient showed motor weakness; Medical research council (MRC) grade 1/1 and spasticity in both upper and lower limbs. Sensory modality was normal, including pain, temperature, joint position, and vibration sense in feet and perineal region. Neck stiffness and Kernig's sign were observed. Deep tendon reflexes were mildly hyper-reflexed. Anal sphincter and bulbocarvenosus reflex were intact. According to the blood analysis, hemoglobin was 10.1 g/L, blood urea nitrogen was 54 mg/dL, and serum creatinine was 9.39 mg/dL. The white blood cell count, platelet count, bleeding time, prothrombin time, activated partial thromboplastin time, homocysteine, protein S, protein C antithrombin III, fibrinogen, and d-dimer were within the normal limits. Anticardiolipin antibody, lupus anticoagulant, and activated protein C resistance were negative. MRI of the cervical region at admission revealed a ventral subdural hematoma distributed from the level of C1 down to T3, compressing the spinal cord (Fig. 1). The lesion was hyperintense on the T2-weighted and isointense T1-weighted images. Brain CT imaging, excluding only associated cerebral hemorrhage, revealed old cerebral infarction in pons and ischemic change in bilateral periventricular white matter.
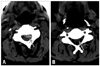
Conservative management with methylprednisolone pulse therapy was administered, despite the extensiveness of the lesion, because of the patient's poor general condition and difficulties associated with general anesthesia and surgery. One g of methylprednisolone per day was intravenously infused for five days. Then he was placed on oral prednisolone 60 mg/d, which was tapered to 20 mg per day over the following two months. Hemodialysis was continued three times per week. The paraplegia of this patient was rapidly recovered after two days of steroid infusion. After the third day of steroid pulse, the patient regained good motor power in both upper and lower limbs (MRC grade 40/40), although he could not walk. After four weeks from first developed weakness, the patient was fully recovered motor weakness (MRC grade 4+/4+). He had minimal spasticity in both lower legs, but did not show bladder nor bowel control problems. The light touch, pain, position, and vibration sensations remained normal. Follow up spine CT scan revealed that subdural hematoma at C3-C4, left paramedian area and other levels were relatively intact (Fig. 2).
Several cases of acute spontaneous subdural hematoma have been published.2-19 They mostly occur in the thoracic spinal area between the ages of 40 and 60 years old. There are no differences in gender. The sudden onset back pain radiating limbs or trunk is frequently observed. Motor, sensory and autonomic deficit are also commonly detected. The time interval from symptom onset to developing neurologic abnormality is various. Moreover, neurologic deficit is presented to multifarious from mild motor or sensory abnormality to severe weakness or bladder dysfunction. In this case, pain and motor deficit occurred, but it was not severe.
A few cases reported cervical involvement only,2-4 and one case had cervical and brain subdural hemorrhage.12 All of these cases were treated with conservative management, however, most of them had poor prognosis except one.4; in one case, the patient showed rapid recovery even though he was treated with methylprednisolone pulse therapy only.
Two articles on spontaneous SSH had previously been reported in our country. One case4 was similar to this case, including cervical involvement, no special treatment and good prognosis. On the other hand, dorsal side hemorrhage was different between them. The other case revealed SSH in thoracic level and treated with lumbar drainage without surgery.6
The spinal MRI revealed iso-signal intensity on T1-weighted images and high signal intensity on T2-weighted images. In the present case, the symptoms developed hyperacutely, and MRI findings supported the hyperacute occurrence. Moreover, hyperacute MRI findings of this case excluded the possibility that systemic heparin employed during hemodialysis two days prior to the onset of symptoms caused SSH.
In the cases presenting acute deterioration and severe neurological deficit in SSH, emergency surgical decompression is generally required. In our case, conservative care was provided since the patient's general condition was very poor, he was not fit for general anesthesia, and the subdural hemorrhage could have been aggravated due to hemodialysis. In this case, we cannot exclude the possibility of spontaneous resolution of SSH. However, the patient showed rapid recovery, and follow up CT scan also revealed improved findings after steroid injection. These findings suggest that the steroid infusion may decrease the mass effect of SSH and assist spontaneous resolution of SSH. Further investigation on steroid treatment for SSH is necessary.
Figures and Tables
References
2. Grobovschek M, Schurich H. [Spinal subdural space-occupying lesions--hematomas]. Rofo. 1989. 150:20–25.
3. Reynolds AF Jr, Turner PT. Spinal subdural hematoma. Rocky Mt Med J. 1978. 75:199–200.
4. Oh SH, Han IB, Koo YH, Kim OJ. Acute spinal subdural hematoma presenting with spontaneously resolving hemiplegia. J Korean Neurosurg Soc. 2009. 45:390–393.

5. Calhoun JM, Boop F. Spontaneous spinal subdural hematoma: case report and review of the literature. Neurosurgery. 1991. 29:133–134.

6. Kim CH, Kim SW, Chang CH, Kim SH. Spontaneous spinal subdural hematoma: treatment with lumbar drainage. J Korean Neurosurg Soc. 2005. 38:481–483.
8. Banach S. [Spontaneous subdural hematoma of the spinal cord]. Neurol Neurochir Pol. 1970. 4:243–246.
9. Boukobza M, Haddar D, Boissonet M, Merland JJ. Spinal subdural haematoma: a study of three cases. Clin Radiol. 2001. 56:475–480.

10. Grunberg A, Carlier R, Bekkali F, Silva M, Chemouilli P, Doyon D. [Spinal subdural hematoma. Presentation of 2 cases studied with MRI]. J Radiol. 1993. 74:291–295.
11. Jacquet G, Godard J, Orabi M, Sönmez S, Steimlé R. Spinal subdural hematoma. Zentralbl Neurochir. 1991. 52:131–135.
12. Jain V, Singh J, Sharma R. Spontaneous concomitant cranial and spinal subdural haematomas with spontaneous resolution. Singapore Med J. 2008. 49:e53–e58.
13. Kang HS, Chung CK, Kim HJ. Spontaneous spinal subdural hematoma with spontaneous resolution. Spinal Cord. 2000. 38:192–196.

14. Küker W, Thiex R, Friese S, Freudenstein D, Reinges MH, Ernemann U, et al. Spinal subdural and epidural haematomas: diagnostic and therapeutic aspects in acute and subacute cases. Acta Neurochir (Wien). 2000. 142:777–785.

15. Kyriakides AE, Lalam RK, El Masry WS. Acute spontaneous spinal subdural hematoma presenting as paraplegia: a rare case. Spine (Phila Pa 1976). 2007. 32:E619–E622.
16. Levy JM. Spontaneous lumbar subdural hematoma. AJNR Am J Neuroradiol. 1990. 11:780–781.
17. Martínez R, Vaquero J, Gilsanz F. Spontaneous spinal subdural hematoma. Case report. J Neurosurg Sci. 1987. 31:157–158.
18. Sakata T, Kurihara A. Spontaneous spinal subdural hematoma. A case report. Spine (Phila Pa 1976). 1984. 9:324–326.
19. Swann KW, Ropper AH, New PF, Poletti CE. Spontaneous spinal subarachnoid hemorrhage and subdural hematoma. Report of two cases. J Neurosurg. 1984. 61:975–980.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download