Abstract
From April 2008 to November 2008, many cases of hepatitis A were reported in Seoul and Gyeonggi Province in Korea. Furthermore, the rate of severe or fulminant hepatitis have significantly increased during the latest epidemic (13.4% vs. 5.2%, p=0.044). Therefore, widespread use of vaccine is warranted to reduce the burden of hepatitis A in Korea.
In Korea, hepatitis A is a disease that must be reported to the district public health authority. Since April 2008, the incidence of hepatitis A has increased, particularly in Seoul and the adjacent areas in Gyeonggi Province (Fig. 1).1 Therefore, we considered this as an epidemic of hepatitis A. At that time, more patients with hepatitis A have been admitted to Severance Hospital, a tertiary teaching hospital in Seoul, Korea (Fig. 2). During this period, many physicians felt that severity, mortality and complications of hepatitis A increased compared with previous period. In the present study, we analyzed clinical features of acute hepatitis A patients who were hospitalized during the latest epidemic. The aim of the present study was to verify an increment of severe cases in recent hepatitis A epidemic and to discuss strategies to prevent hepatitis A in Korea.
We retrospectively reviewed medical records of 245 acute hepatitis A patients who were admitted to Severance Hospital between January 2006 and November 2008, and compared clinical manifestations of hepatitis A observed during the latest epidemic (from April 2008 to November 2008, period 1) with previous one (from January 2006 to March 2008, period 2). Patients who were transferred from other provinces or were suspected to have acquired hepatitis A virus (HAV) in foreign countries were excluded. The diagnosis of acute hepatitis A was made by the detection of serum IgM antibody against HAV along with clinical manifestations. Commercially available enzyme immunoassays (Abbott Laboratories, North Chicago, IL, USA) were used to detect IgM anti-HAV, anti-HCV, HBsAg and antibody to HBsAg. Patients with prothrombin time less than 40% compared to the control and without hepatic encephalopathy were defined as having severe acute hepatitis (AHs).2 Fulminant hepatitis (FH) was defined as less than 40% prothrombin time compared to the control and also presentation of hepatic encephalopathy.3 Limited acute hepatitis (AH) were those with prothrombin time more than 40% compared to the control, and without hepatic encephalopathy. Complications included acute renal failure (ARF), prolonged cholestatic hepatitis, hemolytic anemia, gastrointestinal bleeding, acalculous cholecystitis, pancreatitis and autoimmune hepatitis (antinuclear antibody titer >1:160 or positive smooth-muscle antibody titer >1:80 and compatible liver biopsy findings). ARF was defined as an increase in serum creatinine concentration to ≥0.5 mg/dL or by 50% compared with the baseline value.4 Prolonged cholestatic hepatitis was defined as having clinical jaundice lasting for at least 12 weeks, with a peak of bilirubin >10 mg/dL.5 Continuous data were reported as mean±SD, and the Student's t-test was employed to compare parametric data. Nonparametric data were analyzed with Mann-Whitney U-test. Categorical variables were reported as frequency distributions, and Chi-square or Fisher's exact tests were performed. All p values were two-tailed, and p values <0.05 were considered significant. Statistical analysis was performed using SPSS for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA). This study was approved by the institutional review board of the hospital.
Among our patients, 132 cases of hepatitis A were detected during period 1 (111 cases per 10,000 hospital admissions), and 113 cases during period 2 (30 cases per 10,000 hospital admissions). A total of 224 patients were finally included in this analysis after excluding 21 patients who were referred from other provinces or suspected to have acquired HAV in foreign countries.
The demographic characteristics of patients with hepatitis A are listed in Table 1. The majority of patients were in their thirties (31-40 years, 46.5%) during period 1, whereas the majority of patients were in their twenties (21-30 years, 41.2%) during period 2. Moreover, the rate of patients in teens (11-20 years, 3.9%) during period 1 was greatly reduced compared with period 2 (13.4%). It seems that reduced proportions of age group moved to thirties or forties, suggesting that the pattern of acute hepatitis A is changing from small outbreaks in crowding areas such as school or military, to large-scale outbreaks in community. The majority of patients were males in both period 1 (63.8%) and period 2 (61.9%). Hepatitis A incidence of males was approximately twice higher than that of females. However, there is no evidence to indicate that males are more susceptible to HAV than females.6 The differences between males and females may be related to risk activities such as hand washing and contaminated food ingestion. The underlying disease including hypertension, diabetes and chronic liver diseases were less prevalent in period 1, although it was statistically insignificant. The path of infection was obscure for most patients. The most frequently reported symptoms included myalgia, nausea, vomiting and abdominal pain, and there were no significant differences between the two groups (Table 2). The laboratory tests (leukocyte and platelet counts, hemoglobin, serum aspartate transaminase, alanine tranasaminase, bilirubin and albumin) on admission day or at its worst values did not show significant differences between the two groups (Table 3). However, C-reactive protein at the time of admission was slightly higher in period 1 (2.73±3.26 vs. 1.56±1.86, p=0.012. The clinical complications and outcomes are shown in Table 4. Sixteen patients (11 patients in period 1 and 5 patients in period 2) developed 18 complications associated with hepatitis A. One patient in period 1 had ARF with hemolytic anemia, and one patient in period 2 experienced ARF and gastrointestinal bleeding simultaneously. No patient died during both periods. The proportion of severe cases (severe or fulminant hepatitis A) was significantly higher in period 1 (13.4 vs. 5.2%, p=0.044). However, the duration of admission, the rate of complications and mortality did not increase. Looking at the age distribution of severe cases, severe cases are limited to patients in their twenties or thirties. Particularly, during period 1, more than 10% of patients in these age groups experienced severe forms of hepatitis. Liver biopsy was performed in two patients, and both of them showed portal inflammation and bridging necrosis, compatible with cholestatic hepatitis.
During the recent decades, the incidence of hepatitis A in Korea has decreased along with rapidly improved sanitation and living standards, thanks to the nation's economic growth.7 In Korea, the immunity to HAV in adults over the age of 30 years was 100% in the 1980s and more than 97% in 1999.8 Therefore, acute hepatitis A in adults was considered uncommon, and testing for anti-HAV antibody in adults was not recommended, even in high risk patients. As hygiene conditions improved, transmission factors have been under control, keeping children from being exposed to the virus. Such children became a large adult population with no immunity to HAV. The seroprevalence of anti-HAV has been steadily declined among children. Low rates of IgG anti-HAV have been observed, particularly in the age group of 10-24 years, which matches with the age group of acute hepatitis A patients who were identified through the recently increased incidence in Korea.9 Since the seroprevalence of anti-HAV has been decreasing among adults in Korea,10,11 there is a substantial potential for severe morbidity and mortality during sporadic outbreaks. In this study, the highest incidence was observed in patients in their twenties or thirties, and this was concordant with previous reports published in Korea.10-12 Once they are infected, they develop severe hepatitis.7,13-15 In this study, patients in these age groups are likely to experience severe symptoms. These changes have an impact on morbidity because the severity of symptoms increases with the age of the infected individual. Thus, as natural immunity in the population decreases, particularly in children and young adults, the number of susceptible individuals increases, enhancing the likelihood of hepatitis A outbreaks. A large and increasing number of hepatitis A patients in Korea can be attributable to the increased susceptibility of the population, especially in young people.
Another possible reason is the increased virus circulation in the community, leading to community-wide spread of infection and outbreaks in different groups through various transmission routes. Transportation of goods and people from highly endemic areas has introduced a spread of HAV virus. Genotyping of the virus has not yet been performed during period 1, but is planned. Until the late 1990s, a single HAV genotype IA strain has been detected in Korea. Recently, a new genotype IIIA was found to co-circulate with genotype IA strains in Korea. Moreover, it has been suggested that more various types of strains might have been imported from highly endemic countries.16 Similar epidemiological changes have been seen in many countries worldwide.17 Recent hepatitis A epidemics in urban areas of industrialized countries have not been traced to a common source.18-20 Thus, effective prevention of HAV infection has become increasingly important. Similarly, we were unable to identify risk factors for transmission in more than 75% of patients.
Although the majority of hepatitis A cases are self-limited acute hepatitis, some develop into severe forms of hepatitis or cause unexpected complications.21 Extrahepatic complications occurred in 10 patients during the recent epidemic. The hepatitis A virus may serve as a trigger for autoimmune hepatitis in genetically susceptible people. However, there was no case of autoimmune hepatitis associated with hepatitis A in this study. Fulminant hepatitis, which occurs in less than 1% of the cases, appears to be more frequent in adults than in children.22,23 Previously, disease severity was considered to be linked to individual host factors such as age and underlying liver disease. Older age is classically related to greater morbidity and mortality of hepatitis A. However, it is not clear whether this is due directly to liver failure or to decompensation caused by underlying diseases.24 In the case of the Shanghai epidemic, factors that contributed to mortality in patients with severe diseases included age of >40 years and other comorbid conditions (e.g., chronic hepatitis C).25 In this study, however, patients with underlying liver disease or age of >40 years did not show differences in clinical outcomes. Although more patients were in severe conditions during the latest epidemic, we did not observe any change in mortality. It is quite likely due to the fact that some mortality cases transferred from other district were excluded from this analysis. Although mortality was not significantly different between the two periods, more patients in period 1 experienced severe hepatitis and more patients were hospitalized. Therefore, the morbidity of hepatitis A in the period 1 is not negligible.
Hepatitis A vaccine has not been routinely recommended as part of the childhood immunization scheme in Korea. Vaccination has been recommended for people in high risk groups, including travelers to areas where hepatitis A is endemic, people with occupational exposure to the virus, injection drug users, patients with chronic liver disease, homosexual individuals, and people who live in communities with cyclic epidemics. A need for more widespread use of hepatitis A vaccine is growing in countries with transitional epidemiology such as Korea. In Korea, vaccination of people with high risks is recommended, but not refunded by the public health system. Immunoglobulin such as post-exposure prophylaxis has not been widely used in this country. However, many people who were hospitalized for severe hepatitis A were young and did not have related risk factors. Implementing the current recommendations for vaccination would not have prevented most of complications and deaths occurred during period 1. Without measures that increases immunity to hepatitis A in young adults, future epidemics with severe consequences such as the one described herein, should be expected.
The limitation of the study is relatively small population. Therefore, further research needs to be conducted with a large cohort of patients, in order to properly document complications and management of the disease. Our findings may not be generalized, considering biases related to which patients sought medical care and which physicians chose to hospitalize them. We were unable to assess patients who were not hospitalized. We could not apply a molecular epidemiologic approach due to limitation of samples. Most patients were reported with obscure etiology, and we could not identify transmission route in this study. It could be explained as one of the characteristics of recent urban epidemic, however, it could also be one of limitations of our study related to retrospective nature.
In conclusion, although the majority of hepatitis A patients completely recovered during period 1, many patients had to be hospitalized or developed associated complications. Hepatitis A may become a major public health issue in years to come. Changes in epidemiological pattern would increase the disease burden, may cause large-scale community-wide outbreaks and result in higher healthcare costs. Therefore, prevention of the disease requires not only strengthening young adult's immunity to hepatitis A through vaccination, but also conducting intensive public awareness campaigns and detailed interventions in the course of transmission.
Figures and Tables
Table 3
Biochemical Characteristics of Hepatitis A Patients
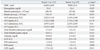
WBC, white blood cell; AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; CRP, C-reactive protein; AH, limited acute hepatitis; AHs, severe acute hepatitis; FH, fulminant hepatitis.
Values are presented as mean±standard deviation.
*Values by Student's t-test or Mann-Whitney U-test.
References
2. Takahashi Y, Shimizu M. The Study Group of Fulminant Hepatitis. Aetiology and prognosis of fulminant viral hepatitis in Japan: a multicentre study. J Gastroenterol Hepatol. 1991. 6:159–164.

3. Fujiwara K, Yokosuka O, Ehata T, Saisho H, Saotome N, Suzuki K, et al. Association between severity of type A hepatitis and nucleotide variations in the 5' non-translated region of hepatitis A virus RNA: strains from fulminant hepatitis have fewer nucleotide substitutions. Gut. 2002. 51:82–88.

5. Gordon SC, Reddy KR, Schiff L, Schiff ER. Prolonged intrahepatic cholestasis secondary to acute hepatitis A. Ann Intern Med. 1984. 101:635–637.

6. Barros H, Oliveira F, Miranda H. A survey on hepatitis A in Portuguese children and adolescents. J Viral Hepat. 1999. 6:249–253.

7. Sohn YM, Rho HO, Park MS, Park JH, Choi BY, Ki M, et al. The changing epidemiology of hepatitis A in children and the consideration of active immunization in Korea. Yonsei Med J. 2000. 41:34–39.

8. Choi W, Eom HS, Kim IH, Lee DH, Kim PS, Kim HG, et al. Patterns of acute hepatitis A and anti-HAV seroprevalence of kyungin province. Korean J Gastroenterol. 1999. 34:69–75.
9. Kim TY, Sohn JH, Ahn SB, Son BK, Lee HL, Eun CS, et al. [Comparison of recent IgG anti-HAV prevalence between two hospitals in Seoul and Gyeonggi area]. Korean J Hepatol. 2007. 13:363–369.

10. Song HJ, Kim TH, Song JH, Oh HJ, Ryu KH, Yeom HJ, et al. Emerging need for vaccination against hepatitis A virus in patients with chronic liver disease in Korea. J Korean Med Sci. 2007. 22:218–222.

11. Lee D, Cho YA, Park Y, Hwang JH, Kim JW, Kim NY, et al. Hepatitis a in Korea: epidemiological shift and call for vaccine strategy. Intervirology. 2008. 51:70–74.

12. Lee EJ, Kwon SY, Seo TH, Yun HS, Cho HS, Kim BK, et al. [Clinical features of acute hepatitis A in recent two years]. Korean J Gastroenterol. 2008. 52:298–303.
13. Rakela J, Redeker AG, Edwards VM, Decker R, Overby LR, Mosley JW. Hepatits A virus infection in fulminant hepatitis and chronic active hepatitis. Gastroenterology. 1978. 74:879–882.

14. Poovorawan Y, Theamboonlers A, Sinlaparatsamee S, Chaiear K, Siraprapasiri T, Khwanjaipanich S, et al. Increasing susceptibility to HAV among members of the young generation in Thailand. Asian Pac J Allergy Immunol. 2000. 18:249–253.
15. Byun KS, Kim JH, Song KJ, Baek LJ, Song JW, Park SH, et al. Molecular epidemiology of hepatitis A virus in Korea. J Gastroenterol Hepatol. 2001. 16:519–524.

16. Yun H, Kim S, Lee H, Byun KS, Kwon SY, Yim HJ, et al. Genetic analysis of HAV strains isolated from patients with acute hepatitis in Korea, 2005-2006. J Med Virol. 2008. 80:777–784.

17. Jenson HB. The changing picture of hepatitis A in the United States. Curr Opin Pediatr. 2004. 16:89–93.

18. Shaw FE Jr, Sudman JH, Smith SM, Williams DL, Kapell LA, Hadler SC, et al. A Community-wide epidemic of hepatitis A in Ohio. Am J Epidemiol. 1986. 123:1057–1065.

19. Skinner JT. Community-wide epidemic of hepatitis A--Shelby County, Tennessee. J Tenn Med Assoc. 1995. 88:468–469.
20. Tong MJ, el-Farra NS, Grew MI. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis. 1995. 171:Suppl 1. S15–S18.

22. Lemon SM, Shapiro CN. The value of immunization against hepatitis A. Infect Agents Dis. 1994. 3:38–49.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download