Abstract
Purpose
Granulocyte colony stimulating factor (G-CSF) has been known to increase neutrophil production and have anti-inflammatory properties, but the effect of G-CSF on pulmonary system is in controversy. We investigated whether G-CSF treatment could attenuate hyperoxia-induced lung injury, and whether this protective effect is mediated by the down-modulation of inflammatory responses in a neonatal rat model.
Materials and Methods
Newborn Sprague-Dawley rats (Orient Co., Seoul, Korea) were subjected to 14 days of hyperoxia (90% oxygen) beginning within 10 h after birth. G-CSF (20 µg/kg) was administered intraperitoneally on the fourth, fifth, and sixth postnatal days.
Results
This treatment significantly improved hyperoxia-induced reduction in body weight gain and lung pathology such as increased mean linear intercept, mean alveolar volume, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling positive cells. Hyperoxia-induced activation of nicotinamide adenine dinucleotide phosphate oxidase, which is responsible for superoxide anion production, as evidenced by upregulation and membrane translocation of p67phox was significantly attenuated after G-CSF treatment, as were inflammatory responses such as increased myeloperoxidase activity and mRNA expression of transforming growth factor-β. However, the attenuation of other proinflammatory cytokines such as tumor necrosis factor-α and interleukin-6 was not significant.
Despite recent improvements in neonatal and perinatal medicine, bronchopulmonary dysplasia (BPD) continues to represent a major cause of mortality and morbidity among premature infants.1 BPD occurs in a growing premature lung, the growth of alveoli is arrested, and the interstitium is thickened, resulting in decrease of surface area for gas exchange. Infants with BPD are prone to respiratory tract infection, asthma, and respiratory decompensation, and outcome of growth impairment and poor neurodevelopment later in life.2,3 There are few effective treatments available for preventing or ameliorating this common and serious disorder. Postnatal dexamethasone has been administered as the sole treatment in patients with BPD. However, it is not recommended for routine use to prevent or treat BPD in preterm infants because of short-term complications and long-term neurodevelopmental effects.4 Two phase III clinical trials for preventions of BPD with inhaled nitric oxide followed by scheduled doses of late surfactant are presently ongoing.5,6 Therefore, the development of a new therapeutic modality to improve the prognosis of this disease is an urgent subject.
Although the pathogenesis of BPD has not been completely delineated, inflammatory responses mediated by neutrophils and proinflammatory cytokines are believed to play a key role in the lung injury process, leading to the development of BPD.7 Therefore, investigation of the effectiveness of new therapeutic modalities with anti-inflammatory capabilities in the treatment of BPD is of a great interest.
Granulocyte colony stimulating factor (G-CSF) is a hematopoietic growth factor that stimulates the production and function of neutrophils.8 Since neutrophils are involved in tissue destruction during inflammatory diseases,9 there is a concern that G-CSF induced neutrophilia might aggravate inflammatory tissue damage. However, G-CSF has also been shown to have anti-inflammatory properties.10 This unique combination of improved production and function of neutrophils and anti-inflammatory effects indicates that G-CSF may be effective for the treatment of various inflammatory conditions.11 Furthermore, G-CSF is one of the few growth factors that have been approved for clinical use to treat neutropenia in newborn infants.12 Therefore, any favorable experimental results can readily be translated into clinical practice. In the pulmonary system, G-CSF has been shown to variously aggravate,13 exhibit neutral effects on,14 or attenuate15 lung injuries. Overall, the available data about the role of G-CSF in the development of lung injury remain largely controversial, and further studies are necessary for clarification.
In the present study, we investigated whether G-CSF treatment could attenuate hyperoxia-induced lung injury, and if so, whether this protective effect is mediated by down-modulation of the inflammatory responses in neonatal rats. Lung injuries were evaluated by morphometric analyses such as mean linear intercept (MLI) and mean alveolar volume, and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining. The extent of inflammatory responses was evaluated by measuring myeloperoxidase (MPO) activity and mRNA expression of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and transforming growth factor-β (TGF-β). Nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) activation which was represented by membrane translocation of p67phox was measured to evaluate the production of reactive oxygen species (ROS).
The experimental protocols described herein were reviewed and approved by the Animal Care and Use Committee of Samsung Biomedical Research Institute, Seoul, Korea. This study was also performed in accordance with institutional and National Institutes of Health (NIH) guidelines for laboratory Animal Care. Timed pregnant Sprague-Dawley rats (Orient Co., Seoul, Korea) were housed in individual cages with free access to water and laboratory chow. Rat pups were delivered spontaneously and reared with their dams. The experiment began within 10 h after birth and continued through postnatal day (P) 14.
Rat pups were randomly divided into four groups with six to eight animals in each group: normoxia control group (NC); normoxia with G-CSF treatment group (NG); hyperoxia control group (HC); and hyperoxia with G-CSF treatment group (HG). Rat pups in the NC and NG groups were kept with nursing mothers in standard cages and normal room air throughout the experiment, and pups in the HC and HG groups were maintained with nursing mothers in standard cages within 50 L Plexiglas chambers in which an oxygen concentration of 90% was maintained. The humidity and environmental temperature were maintained at 50% and 24℃, respectively. Nursing mother rats were rotated daily between litters in the normoxia and hyperxoxia groups to avoid oxygen toxicity.
Rat pups in the NG and HG groups were injected intraperitoneally with 20 µg/kg of recombinant human G-CSF (Dong-A Pharmaceutical Co. Ltd., Seoul, Korea) in 5% dextrose solution (2 µg/mL) on P 4, 5 and 6. Pups in the NC and HC groups received the same volume of 5% dextrose solution injected in the same manner. The survived rat pups in each group were weighed daily until sacrifice under deep pentobarbital anesthesia (60 mg/kg, intraperitoneal) at P 14. Morphometric and biochemical analyses of whole lung tissue was done.
For biochemical analyses, transcardiac perfusion using ice-cold phosphate buffered saline (PBS) was done, and then lungs were resected, snap-frozen in liquid nitrogen, and refrigerated at - 80℃.
Lungs were inflated by instillation of 10% buffered formalin with a constant pressure of 20 cm H2O, and fixed at room temperature through the night for morphometric analyses. Then the fixed lung tissue was processed, and embedded in paraffin. Four-micrometer-thick sections of paraffin blocks were stained with hematoxylin and eosin. Images of each field were captured using a digital camera through an Olympus BX40 microscope (Olympus Optical Co. Ltd., Tokyo, Japan).
MLI and mean alveolar volume were measured to evaluate the level of alveolarization. The mean inter-alveolar distance was measured as MLI, by dividing the total length of the lines drawn across the lung section by the number of intercepts encountered, as described by Thurlbeck.16 The mean alveolar volume was calculated using the method reported by Snyder, et al.17 Briefly, a grid containing equally spaced crosses was placed on a uniformly enlarged photomicrograph of each lung field. The diameters (ℓ) of the alveoli containing crosses were measured along the horizontal axes of the crosses. The cube of the alveolar diameter times π and divided by 3 (ℓ3π/3) was used to estimate the mean alveolar volume. A minimum of two sections per rat and six fields per section were selected randomly and examined for each analysis. Analyses were carried out by two independent observers who were blind to treatment conditions.
Immunofluorescent TUNEL staining (kit S7110 ApopTag, Chemicon, Temecula, CA, USA) was done to determine the extent of apoptosis in the lung. Deparaffinized and rehydrated paraffin section slides were digested for 15 min using proteinase K (20 µg/mL in PBS) (Sigma Co., St. Louis, MO, USA) at room temperature, washed for 10 min with PBS. Sections were incubated using working strength TdT enzyme at 37℃ for 1 h after incubated using equilibration buffer for 1 min, and then immersed in a stop/wash buffer and rinsed. The sections tagged with fluorescein isothiocyanate (FITC)-labeled anti-digoxigenin conjugate were incubated at room temperature for 30 min in the dark, and propidium iodide (0.5 µg/mL, Sigma Co.) was used for nuclear counterstaining. Slides were mounted with a Vectashield mounting solution (Vector Laboratories, Burlingame, CA, USA), and visualized by fluorescent microscopy (Nikon E600 fluorescence microscope, Tokyo, Japan). The number of TUNEL positive cells were counted in ten non-overlapping fields with a magnifying power of ×200.
The MPO activity which is an indicator of neutrophil accumulation, was made by modification of the method reported by Gray, et al.18 The homogenized lung tissues in a phosphate buffer (pH 7.4) were centrifuged at 30,000 g for 30 min, resuspended in another phosphate buffer with 0.5% hexadecyltrimethyl ammonium bromide (50 mM, pH 6.0), and measured absorbance changes at 460 nm, using 0.167 mg/mL of O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide to assay the activity of MPO. One unit of MPO activity equaled the amount of enzyme to degrade 1 µmole of peroxide per minute.
The total RNA in the sample was extracted by using a RNA Trizol kit (Invitrogen Corporation, Carlsbad, CA, USA) according to the manufacturer's protocol. One microgram of RNA was used to produce cDNA with an Protoscript® II RT-PCR kit (New England Biolabs, Ipswich, MA, USA). PCR primers for rat TNF-α, IL-6, TGF-β and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed with Primer 3 (Whitehead Institute, Cambridge, MA, USA) and were synthesized by Bioneer Inc. (Bioneer, Daejeon, Korea). The sequence of primers used was as follows: (TNF-α) sense 5'-TACTGAACTTCGGGGTGATCGGTCC-3', antisense 5'-CAGCCTTGTCCCTTGAAGAGAACC-3' (IL-6) sense 5'-CACCAGGAACGAAAGTCAACTC-3', antisense 5'-GGAAGCATCCATCATTTCTTTG-3' (TGF-β) sense 5'-CAACTGTGGAGCAACACGTAGA-3', antisense 5'-CAACCCAGGTCCTTCCTAAAGT-3' (GAPDH) sense 5'-CTCTACCCACGGCAAGTTCAA-3', antisense 5'-GGGATGACCTTGCCCACAGC-3'.
One µL of cDNA was mixed in 19 µL of reaction mixture of PCR Master Mix (Bioneer, Daejeon, Korea) and then 0.5 µmole of each primer (Bioneer) was added for each reactions which were carried out in a T1 thermocycler (Biometra, Goettingen, Niedersachsen, Germany). The cycle profile involved was as follows: 60 s at 95℃, 60 s at X℃, where X is the annealing temperature for each pair of cytokine primers, and 60 s at 68℃. The PCR products were visualized by ethidium bromide after separated using 1.2% agarose gel electrophoresis. Then they were scanned by a Gel Doc 2000 analyzer (Bio-Rad Laboratories, Hercules, CA, USA). The mRNA level of TNF-α, IL-6, and TGF-β were analyzed densitometrically with Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA), which were estimated from the density ratio of cytokines to GAPDH (control).
p67phox is a subunit of NADPH oxidase, the translocation of which from the cytosol to the plasma membrane generates reactive oxygen species (ROS) and reflects NADPH oxidase activation.19 Lung tissues were fractionated into membrane and cytosolic components for Western blot analysis. Homogenized tissues in ice-cold hypertonic solution were centrifuged at 600 g for 10 min, and the supernatant was centrifuged at 100,000 g for 1.5 h. Then the supernatant containing both the cytosolic and the membrane-particulate pellet was resuspended in hypotonic solution containing 1% Triton X-100. Western blot analysis of these products using antibodies against the p67phox (1 : 500, BD Biosciences, San Diego, CA, USA), the NADPH oxidase cytosolic subunit was done, and the bands were recognized by horseradish-peroxidase (HRP)-conjugated anti-mouse secondary antibody (1 : 1,000, DAKO, Glostrup, Denmark). Developed Western blots using enhanced luminol-based chemiluminescent (ECL) detection reagents (Amersham Pharmacia, Uppsala, Sweden) were exposed to X-ray film (Fuji, Tokyo, Japan), and re-probed with antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1 : 1,000, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). The membrane fraction was presented to immunoblotting for calnexin (1 : 500 Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), a membrane marker to determine the relative degree of membrane purification.
The data are expressed as mean ± SEM. Survival curve comparisons were performed using Kaplan-Meier analysis followed by a log rank test. For continuous variables with a normal distribution, ANOVA with a Bonferroni correction was performed. For variables without normal distribution, we performed Wilcoxon rank sum tests with Bonferroni corrections. p values < 0.05 were considered significant.
Exposure to oxygen (HC) reduced the survival rate of the animals to 88% at the end of the experiment (P 14) compared to the 100% survival rate of the normoxia groups (NC and NG), and this reduced survival rate in HC was improved to 92% with G-CSF treatment (HG), however, this improvement was not statistically significant.
Although birth weight was not significantly different between the four experimental groups, body weight at P 14 in HC was significantly lower compared to the normoxia groups, and this decreased body weight gain observed in HC was significantly improved with G-CSF treatment (HG) (Fig. 1).
Representative photomicrographs showing differences between the experimental groups are shown in Fig. 2. Fewer and larger alveoli were observed in HC pups compared to pups in the normoxia groups, indicating hyperoxia-induced impairments in alveolar growth, and these abnormalities seemed to be attenuated with G-CSF treatment (HG). Morphometric data demonstrated significantly increased MLI and mean alveolar volume in HC compared to normoxia groups (NC and NG), and significant attenuation of these abnormalities with G-CSF treatment (HG) support these microscopic findings (Fig. 3).
The numbers of TUNEL positive cells in distal lung samples were markedly increased in HC compared to the normoxia groups (NC and NP), and G-CSF treatment (HG) significantly attenuated this hyperoxia-induced increase in the number of TUNEL positive cells (Fig. 4).
Exposure to oxygen (HC and HG) significantly increased white blood cell counts in the blood in hyperoxia, compared to the normoxia groups (NC and NG), and the G-CSF induced granulopoiesis was not observed at P14 (Fig. 5A).
The significantly increased MPO activity observed in HC, compared to the normoxia groups (NC and NG), was significantly improved with G-CSF treatment (HG) (Fig. 5B).
In semi-quantitative RT-PCR, significantly increased mRNA levels of TNF-α, IL-6 and TGF-β were observed in HC, compared to the normoxia groups (NC and NG) (Fig. 6). The hyperoxia-induced increase in TGF-β mRNA expression was significantly attenuated with G-CSF treatment (HG), however, the attenuation of TNF-α and IL-6 mRNA levels was not statistically significant.
When NADPH oxidase is activated, expression and membrane translocation of a cytosolic subunit of NADPH oxidase p67phox is increased. Hyperoxia-induced production of ROS was evaluated by NADPH oxidase activation, because NADPH oxidase produces oxygen free radicals both in phagocytic20 and nonphagocytic cells.21 Exposure to oxygen (HC) significantly increased p67phox both in the cytosolic and membrane fractions, indicating activation of NADPH oxidase, compared to the normoxia groups (NC and NG) in Western blot analyses, and the G-CSF (HG) significantly attenuated this hyperoxia-induced NADPH oxidase activation (Fig. 7).
In the present study, prolonged exposure of newborn rat pups to hyperoxia for two weeks increased mortality, retarded growth, and led to the development of lung injuries similar to those seen in premature human infants with BPD.22 These pups exhibited decreased alveolarization, as shown by increased MLI and alveolar volume,23 and significantly increased TUNEL positive cells.24 G-CSF treatment significantly attenuated hyperoxia-induced growth retardation, decreased alveolarization and increased TUNEL positive cells in the newborn rats.
G-CSF is clinically available for use in neonates,12 and its safety and feasibility have been validated in recent human stroke therapy trials.11 Taken together, these findings suggest that G-CSF might be a useful new therapeutic agent for hyperoxia-induced neonatal lung diseases such as clinical BPD, for which no effective treatments are currently available. However, better understanding of its mechanism of action must be obtained for translation of this experimental result to clinical trials.
We observed significant increases in MPO activity and mRNA of TNF-α, IL-6 and TGF-β in rat pups in the HC group, suggesting that inflammation mediated by neutrophils7 and proinflammatory cytokines25 plays a central role in the development of BPD.26 G-CSF caused not only a significant attenuation of hyperoxia-induced increase in MPO activity, but also caused a similar decrease in hyperoxia-induced increase in TGF-β. These results suggest that the protective effects of G-CSF against hyperoxic neonatal lung injury are associated with or mediated by anti-inflammatory effects. The anti-inflammatory action of G-CSF has been reported also in various experimental and clinical conditions including murine endotoxemia,27 encephalitis, non-neutropenic surgical intensive care patients,28 pneumonia29 and stroke.30 Although G-CSF suppressed the synthesis of proinflammatroy cytokines such as TNF-α both in vitro and in vivo,27 and upregulated the production of anti-inflammatory mediators such as soluble TNF-α receptor type I and II, IL-6, IL-10 and IL-1 receptor antagonist,15,31 the precise mechanisms of its anti-inflammatory action are not yet clear. Further studies are necessary to clarify this action.
Neutrophils have been known to play a role in the exacerbation of prior lung injury with G-CSF treatment.13 However, in the present study G-CSF treatment significantly attenuated lung MPO activity, and did not induce leukocytosis. The extent of leukocytosis with G-CSF treatment has been known to be dose-dependent.13 The dose of G-CSF used in this study was similar to that prescribed clinically32,33 and relatively low compared to other studies.34,35 Therefore, besides differences in animal species and experimental design, different doses of G-CSF might be responsible for these conflicting results. Further studies are needed to determine the optimal dosage.
Oxidative stress to the immature lung is a major contributing factor for the development of BPD.36 NADPH oxidase is responsible for superoxide anion production (O2-), which generates other ROS such as hydrogen peroxide, hydroxyl radical and hypochlorous acid.20 As the cytoplasmic subunits p47phox, p67phox, p40phox and Rac translocate to membrane-bound cytochrome upon activation of this multicomponent enzyme complex NADPH oxidase,37 membrane translocation of the p67phox can be served as an in vivo indicator of NADPH oxidase activation as shown in our previous study.38
In the present study, increased expression of the p67phox protein was observed both in the cytosolic and membrane fraction in HC, and G-CSF treatment significantly attenuated hyperoxia-induced NADPH oxidase activation as evidenced by reduced membrane translocation of the p67phox in the lung tissue. As phagocytic NADPH oxidase activation seems to be the main source of ROS production in the lung,39 significant attenuation of hyperoxia-induced increase in lung MPO activity, an indicator of the lung neutrophil accumulation, with G-CSF treatment might be responsible for its anti-oxidative effects.
In summary, G-CSF treatment significantly attenuated hyperoxia-induced lung injuries such as decreased alveolarization and increased TUNEL positive cells by down-modulating the oxidative stress and inflammatory responses in neonatal rats. These findings suggest the potential use of G-CSF as a new therapeutic agent for BPD. Further studies regarding optimal dosage, timing and safety in multi-species preterm and term neonates will be necessary for the translation of the benefits of G-CSF treatment observed in this study to clinical trials. In addition, neurological studies have to be done when neonates administered with G-CSF neonatally become adults.
Figures and Tables
 | Fig. 1Daily body weight gain in each experimental group. Data; mean ± SEM. NC, normoxia control group (n = 12); NG, normoxia with G-CSF treatment group (n = 12); HC, hyperoxia control group (n = 14); HG, hyperoxia with G-CSF treatment group (n = 14). *p < 0.05 compared to NC. †p < 0.05 compared to HC. |
 | Fig. 2Representative optical photomicrographs of P 14 rat lungs stained with hematoxylin and eosin. Scale bar = 100 µm, NC, normoxia control group; NG, normoxia with G-CSF treatment group; HC, hyperoxia control group; HG, hyperoxia with G-CSF treatment group. |
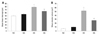 | Fig. 3Comparisons of alveolarization measured by the mean linear intercept (A) and mean alveolar volume (B). Data; mean ± SEM. NC, normoxia control group (n = 6); NG, normoxia with G-CSF treatment group (n = 6); HC, hyperoxia control group (n = 8); HG, hyperoxia with G-CSF treatment group (n = 8). *p < 0.05 compared to NC. †p < 0.05 compared to HC. |
 | Fig. 4TUNEL positive cells (A) and number of TUNEL positive cells (B) in P14 rat lungs. TUNEL positive cells labeled with FITC (green, arrow) and the nuclei labeled with DAPI (blue) (Scale bar, 25 µm) (A). Data; mean ± SEM. NC, normoxia control group (n = 6); NG, normoxia with G-CSF treatment group (n = 6); HC, hyperoxia control group (n = 8); HG, hyperoxia with G-CSF treatment group (n = 8). *p < 0.05 compared to NC. †p < 0.05 compared to HC. TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling; FITC, fluorescein isothiocyanate; DAPI, 4, 6-diamidino-2-phenylindole; G-CSF, granulocyte colony stimulating factor. |
 | Fig. 5White blood cell count in blood (A) and myeolperoxidase activity in P 14 rat lungs (B). Data; mean ± SEM. NC, normoxia control group (n = 6); NG, normoxia with G-CSF treatment group (n = 6); HC, hyperoxia control group (n = 6); HG, hyperoxia with G-CSF treatment group (n = 6). *p < 0.05 compared to NC. †p < 0.05 compared to HC. MPO, myeloperoxidase. |
 | Fig. 6Representative RT-PCR blots and densitometric histograms for TNF-α, IL-6, and TGF-β. NC, normoxia control group (n = 6); NG, normoxia with G-CSF treatment group (n = 6); HC, hyperoxia control group (n = 6); HG, hyperoxia with G-CSF treatment group (n = 6). *p < 0.05 compared to NC. †p < 0.05 compared to HC. RT-PCR, reverse transcriptase polymerase chain reaction; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; TGF-β, transforming growth factor-β; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. |
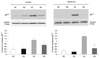 | Fig. 7Western blot analysis of p67phox in the cytosol and membrane fractions of P 14 rat lung. NC, normoxia control group (n = 6); NG, normoxia with G-CSF treatment group (n = 6); HC, hyperoxia control group (n = 6); HG, hyperoxia with G-CSF treatment group (n = 6). *p < 0.05 compared to NC. †p < 0.05 compared to HC. |
ACKNOWLEDGEMENTS
This work was supported by the IN-SUNG Foundation of Medical Research (C-A7-840-1).
References
2. Short EJ, Klein NK, Lewis BA, Fulton S, Eisengart S, Kercsmar C, et al. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003. 112:e359.

3. Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005. 116:1353–1360.

4. Committee on Fetus and Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002. 109:330–338.
5. Trial of late surfactant for prevention of bronchopulmonary dysplasia (TOLSIRF). from: URL: http://www.clinicaltrials.gov/ct2/show/nct01022580.
6. Trial of late surfactant to prevent BPD: A pilot study in ventilated preterm neonates receiving inhaled nitric oxide (TOLSURF Pilot). from: URL: http://www.clinicaltrials.gov/ct2/show/nct00569530.
7. Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med. 2006. 11:354–362.

8. Juul S, Felderhoff-Mueser U. Epo and other hematopoietic factors. Semin Fetal Neonatal Med. 2007. 12:250–258.

10. Boneberg EM, Hartung T. Molecular aspects of anti-inflammatory action of G-CSF. Inflamm Res. 2002. 51:119–128.

11. Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004. 110:1847–1854.

12. Carr R, Modi N, Doré C. G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst Rev. 2003. CD003066.

13. Adach K, Suzuki M, Sugimoto T, Suzuki S, Niki R, Oyama A, et al. Granulocyte colony-stimulating factor exacerbates the acute lung injury and pulmonary fibrosis induced by intratracheal administration of bleomycin in rats. Exp Toxicol Pathol. 2002. 53:501–510.

14. Miura G, Awaya H, Matsumoto T, Tanaka N, Matsunaga N. Does granulocyte colony-stimulating factor exacerbate radiation-induced acute lung injury in rats? Radiat Med. 2000. 18:227–232.
15. Wiedermann FJ, Mayr AJ, Hobisch-Hagen P, Fuchs D, Schobersberger W. Association of endogenous G-CSF with anti-inflammatory mediators in patients with acute respiratory distress syndrome. J Interferon Cytokine Res. 2003. 23:729–736.

16. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1--postnatal lung growth. Thorax. 1982. 37:572–579.

17. Snyder JM, Jenkins-Moore M, Jackson SK, Goss KL, Dai HH, Bangsund PJ, et al. Alveolarization in retinoic acid receptor-beta-deficient mice. Pediatr Res. 2005. 57:384–391.

18. Gray KD, Simovic MO, Chapman WC, Blackwell TS, Christman JW, May AK, et al. Endotoxin potentiates lung injury in cerulein-induced pancreatitis. Am J Surg. 2003. 186:526–530.

20. Robinson JM, Badwey JA. The NADPH oxidase complex of phagocytic leukocytes: a biochemical and cytochemical view. Histochem Cell Biol. 1995. 103:163–180.

21. Höhler B, Holzapfel B, Kummer W. NADPH oxidase subunits and superoxide production in porcine pulmonary artery endothelial cells. Histochem Cell Biol. 2000. 114:29–37.

22. Bland RD. Neonatal chronic lung disease in the post-surfactant era. Biol Neonate. 2005. 88:181–191.

23. Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, et al. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2005. 289:L529–L535.

24. McGrath-Morrow SA, Stahl J. Apoptosis in neonatal murine lung exposed to hyperoxia. Am J Respir Cell Mol Biol. 2001. 25:150–155.

25. Chanock SJ, el Benna J, Smith RM, Babior BM. The respiratory burst oxidase. J Biol Chem. 1994. 269:24519–24522.

27. Görgen I, Hartung T, Leist M, Niehörster M, Tiegs G, Uhlig S, et al. Granulocyte colony-stimulating factor treatment protects rodents against lipopolysaccharide-induced toxicity via suppression of systemic tumor necrosis factor-alpha. J Immunol. 1992. 149:918–924.
28. Gross-Weege W, Weiss M, Schneider M, Wenning M, Harms B, Dumon K, et al. Safety of a low-dosage Filgrastim (rhG-CSF) treatment in non-neutropenic surgical intensive care patients with an inflammatory process. Intensive Care Med. 1997. 23:16–22.

29. Knapp S, Hareng L, Rijneveld AW, Bresser P, van der Zee JS, Florquin S, et al. Activation of neutrophils and inhibition of the proinflammatory cytokine response by endogenous granulocyte colony-stimulating factor in murine pneumococcal pneumonia. J Infect Dis. 2004. 189:1506–1515.

30. Shyu WC, Lin SZ, Lee CC, Liu DD, Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ. 2006. 174:927–933.

31. Hartung T, Döcke WD, Gantner F, Krieger G, Sauer A, Stevens P, et al. Effect of granulocyte colony-stimulating factor treatment on ex vivo blood cytokine response in human volunteers. Blood. 1995. 85:2482–2489.

32. Donini M, Fontana S, Savoldi G, Vermi W, Tassone L, Gentili F, et al. G-CSF treatment of severe congenital neutropenia reverses neutropenia but does not correct the underlying functional deficiency of the neutrophil in defending against microorganisms. Blood. 2007. 109:4716–4723.

33. Carr R, Brocklehurst P, Doré CJ, Modi N. Granulocyte-macrophage colony stimulating factor administered as prophylaxis for reduction of sepsis in extremely preterm, small for gestational age neonates (the PROGRAMS trial): a single-blind, multicentre, randomised controlled trial. Lancet. 2009. 373:226–233.

34. Minnerup J, Sevimli S, Schäbitz WR. Granulocyte-colony stimulating factor for stroke treatment: mechanisms of action and efficacy in preclinical studies. Exp Transl Stroke Med. 2009. 1:2.

35. Kim BR, Shim JW, Sung DK, Kim SS, Jeon GW, Kim MJ, et al. Granulocyte stimulating factor attenuates hypoxic-ischemic brain injury by inhibiting apoptosis in neonatal rats. Yonsei Med J. 2008. 49:836–842.

36. Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003. 8:39–49.

37. Bastian NR, Hibbs JB Jr. Assembly and regulation of NADPH oxidase and nitric oxide synthase. Curr Opin Immunol. 1994. 6:131–139.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download