Abstract
Purpose
To examine the feasibility of endoscopic thyroidectomy (ET) via an axillo-breast approach without gas insufflation for large thyroid tumors and micropapillary carcinomas.
Materials and Methods
The patients in the benign group were separated into groups 1 (n=95, <4 cm in tumor diameter) and 2 (n=37, ≥4 cm in tumor diameter). Also, 57 patients in the micropapillary carcinoma group underwent an endoscopic hemithyroidectomy (HT) (group 3) and were compared with 60 patients who received conventional open HT (group 4). Postoperative functional outcome, local complications, surgical outcomes, and pathological outcomes were compared between the groups.
Results
In the benign group, there was no significant difference in mean operating time, hospital stay, or overall perioperative complications between the two groups. In the micropapillary carcinoma group, mean operating time and hospital stay in group 3 were significantly longer than in group 4 (p=0.015 and p≤0.001). The overall perioperative complications did not differ significantly between the groups. The postoperative cosmetic result was better in groups 1-3 (endo group) than in group 4 (open group).
Conclusion
ET via a gasless axillo-breast approach seems to be a safe procedure even for benign thyroid lesions ≥4 cm and micropapillary carcinomas. Although it has the advantage of better cosmetic results over open thyroidectomy, there is room for improvement in terms of lessening its invasiveness and shortening the operative time.
Endoscopic thyroid surgery is no longer a new method. Over the past decade, several endoscopic thyroidectomy techniques have been described, with the primary aim of obtaining better cosmetic results. The new methods have largely been developed because the incidence of thyroid nodules is on the rise and all involved parties are concerned about cosmetic results.1-19 Although the indications for endoscopic thyroidectomy have been expanded extensively,20 there has been limited acceptance of their application for large benign thyroid lesions (≥3-4 cm in diameter or ≥20 mL in volume) and for thyroid cancer.2,4,20-22
As the size of a benign thyroid lesion increases, so does the potential necessity for surgical treatment. Furthermore, the prevalence of micropapillary carcinoma has increased, and a significant number of these patients are young women.1,23 Therefore, to gain wider acceptance for endoscopic thyroidectomy, surgical technique and feasibility for the management of large size goiters (≥4 cm in diameter) and endoscopic thyroidectomy including central neck dissection (CND) should be very clearly established.
Our group has previously described a unilateral axillo-breast approach for endoscopic thyroid surgery without gas insufflation.1,24 As we accumulate experience, the practice of endoscopic thyroidectomy within our institute has stabilized, and we have expanded the inclusion criteria to include micropapillary carcinomas and larger benign tumors. This study will review our experience with endoscopic thyroidectomy via an axillo-breast approach without gas insufflation and its applications to large benign tumors (≥4 cm in tumor diameter) and micropapillary carcinomas, considering the basic oncologic principles and biology of thyroid cancer.
The study group comprised patients who underwent endoscopic thyroid surgery for benign thyroid lesions or micropapillary carcinoma without lymph node metastasis at the Department of Otolaryngology-Head and Neck Surgery, Soonchunhyang University College of Medicine, between January 2007 and December 2008. This was a retrospective analysis of the outcomes of endoscopic thyroidectomy surgical as performed by the same surgeon (Y.W.K.) and was not initially intended to be a comparison. The minimum follow-up period was 6 months.
Based on our positive experience with this technique, we have recently begun to expand the indications for endoscopic thyroidectomy to micropapillary carcinoma1 and large size goiters (≥4 cm in diameter). A total thyroidectomy (TT) using the bilateral axillo-breast approach was performed in a limited number of micropapillary carcinoma cases; however, the inclusion criteria were restricted to endoscopic hemithyroidectomy (HT) and TT for benign nodules or endoscopic HT in conjunction with prophylactic ipsilateral CND for micropapillary carcinoma.
We grouped the patients into benign and micropapillary carcinoma groups, depending on the final pathological report. The patients in the benign group were separated into two groups. Group 1 consisted of patients with tumor diameter <4 cm, and group 2 consisted of patients with tumor diameter ≥4 cm. We performed HT or TT according to the bilaterality or multiplicity of thyroid lesions. In most patients, this surgical method was chosen to correct cosmetic disfigurement arising from a noticeably enlarged gland or was performed under the advice of an endocrinologist after unsuccessful medical therapy.
Additionally, in cases of micropapillary carcinoma, endoscopic HT with CND (group 3) and open HT with CND (group 4) were retrospectively analyzed with no initial intention to compare the two. We defined HT as a unilateral thyroid lobectomy plus an isthmusectomy. The central compartment lymph nodes consist of the level 6 lymph nodes (Delphian/prelaryngeal, pretracheal, and paratracheal lymph nodes), which include the lymph nodes from the hyoid bone superior to the suprasternal notch inferiorly. On each side, the lateral boundary is limited by the medial border of the carotid sheath. Ipsilateral CND via the endoscopic approach consisted mostly of ipsilateral paratracheal and pretracheal lymph node dissection. Open HT with CND was chosen by a review of the records of 60 consecutive patients who underwent the conventional open procedure between January 2006 and December 2008.
Our contraindications for endoscopic thyroidectomy included a history of thyroiditis, Graves' disease, previous neck or thyroid surgery, or irradiation. We also excluded cases with micropapillary carcinoma found coincidentally during resection for multinodular goiter. As described in a previous study,1 our indications for HT with ipsilateral CND used to manage papillary thyroid carcinoma were an intra-thyroidal papillary carcinoma smaller than 1 cm in diameter on preoperative sonography, with no evidence of central or lateral lymph node metastasis on preoperative imaging, and unilateral and solitary thyroid lesions only. We excluded patients with a possible extra-thyroidal extension or abutment on the thyroid capsule, especially near the tracheoesophageal groove, on preoperative sonography or computed tomography (CT) even when the lesion was smaller than 1 cm. All patients were evaluated preoperatively via sonography and/or CT and fine-needle aspiration cytology. Thyroid size was calculated by sonography and/or CT. Thyroid function test results were normal in all patients.
Informed consent was not required for this retrospective analysis, but written informed consent was obtained regarding the possible conversion to open surgery or reoperation (completion of thyroidectomy for the contralateral thyroid gland using an open or endoscopic procedure, and lymph node dissection using open surgery), according to the final pathological results. The institutional review board of Soonchunhyang University College of Medicine approved this retrospective study.
The operative technique was performed as previously described.1,24 The patient was placed in the supine position under general anesthesia. The neck was extended slightly and the lesion-side arm was raised to fully expose the axilla. The surgical team consisted of the surgeon and one assistant, who was required to hold a 30° rigid endoscope and a suction-irrigator. A 4.5-5.5 cm skin incision was made parallel to the skin crease in the axillary fossa, through which we inserted the rigid endoscope and the endoscopic instruments (Fig. 1A). The skin was elevated above the pectoralis major muscle exclusively under direct vision, using monopolar cauterization through the axillary skin incision, until the anterior border of the sternocleidomastoid muscle was exposed. To create a working space, we inserted an external retractor (Sejong Medical Corporation, Gyoha, Korea) through the skin incision in the axilla, which was raised using a lifting device. A second 1.0 cm skin incision was made along the upper margin of the mammary areola on the tumor side for inserting a 12 mm trocar, which was directed to the midline of the sternal notch. Using only a harmonic scalpal (HS; Harmonic Ace 36P®; Johnson & Johnson Medical, Cincinnati, OH, USA), we dissected the anterior border of the sternocleidomastoid muscle from the sternohyoid muscle and, in some cases, divided the omohyoid muscle.
The thyroid gland was exposed by dissecting the sternothyroid muscle with the HS and by lateral traction of the sternocleidomastoid muscle. In all cases, only the HS was used for vascular control of the thyroid gland and strap muscles. The superior, middle, and inferior thyroidal arteries and veins were controlled using a low-power level (70 µm vibration) to improve hemostasis. The other small vessels and surrounding connective tissue were controlled using a higher power level (100 µm vibration) for easy cutting. After elevating the skin flap with monoplar cauterization, we used the HS and an endoscopic dissector for vessel sealing and tissue dissection throughout the operation. The upper pole was fully exposed with careful dissection. The superior thyroid vessels were identified and divided close to the thyroid gland using the HS, while avoiding injury to the external branch of the superior laryngeal nerve (Fig. 1B). The superior parathyroid gland was identified during dissection and left intact (Fig. 1C). The lateral view from the axillary port made it easy to observe the upper pole of the thyroid and to divide the vessels. We identified the tracheal wall, exposed it as a midline landmark and dissected the lower pole from the adipose tissue and trachea. During this procedure, care was taken to avoid injury to the tracheal wall with the HS. Next, the inferior thyroid vein was divided close to the thyroid gland using the HS to avoid injury to the inferior parathyroid gland. The thyroid gland was then retracted medially, and the perithyroidal fascia was divided and dissected using an endoscopic dissector and the HS. We performed a careful dissection to identify the inferior thyroid artery and recurrent laryngeal nerve in the tracheoesophageal groove (Fig. 1D). Until now, we have had no experience with a neuron-monitoring system during thyroid surgery. During the endoscopic thyroidectomy procedure, we can easily identify and preserve the recurrent laryngeal nerve (RLN) under magnified surgical view. However we think that further investigations of this system are necessary to make comparisons regarding postoperative RLN status.
The inferior thyroid artery was divided close to the thyroid gland to avoid injury to the recurrent laryngeal nerve (Fig. 2A). During this procedure, the lateral view of the thyroid gland from the axillary port helped to ensure complete preservation and exposure of the nerve (Fig. 1D). Proper application of an endoscopic cottonoid and a dissector to Berry's ligament realized the complete dissection of the thyroid gland from the trachea. To prevent thermal injury, especially near Berry's ligament, we took great care to maintain a distance of at least 5 mm from the major neurovascular structures and the trachea. The thyroid gland was then dissected from the trachea, and the isthmus was resected using the HS.
During ipsilateral CND (the pretracheal and paratracheal area on the tumor side), we dissected close to the recurrent laryngeal nerve using an endoscopic dissector to avoid damage by collateral energy from the HS (Fig. 2B). The CND was performed en bloc with a thyroidectomy (Fig. 2C) or separately after HT. The resected specimen was extracted through the axillary skin incision. In cases of TT, a contralateral endoscopic lobectomy was performed via a contralateral axillo-breast approach. During the entire procedure, we made every effort to perform an en bloc resection without rupturing the tumor (Fig. 2D).
We placed a Jackson-Pratt closed suction drain (200 mL, 3.2 mm diameter; Barovac Sewoon Medical, Seoul, Korea) in the wound and closed it with 4-0 Vicryl (subcutaneous) and 5-0 nylon (skin) sutures.
In all of the conventional open HT cases (group 4), a standard extracapsular resection was used for the thyroidectomy. The HS was used exclusively for vascular control of the thyroid gland. The CND was performed en bloc with a thyroidectomy or separately after HT. The fibrofatty tissue containing numerous nodes was removed within an area limited cranially by the hyoid bone and superior thyroid vessels, caudally by the venous brachiocephalic trunk, and laterally by the medial border of the carotid arteries. In our previous report, we suggest that routine prophylactic drainage should be avoided, even in central neck dissection, if intraoperative hemostasis is ensured, so we did not use a drainage system in the open surgery cases.
The following variables, including perioperative parameters (volume and duration of drainage and hospital stay), local complications, surgery-related outcomes, and pathological outcomes, were compared between the groups. Operating time was defined as the time interval between skin incision and closure. Laryngoscopic examination was used to evaluate vocal cord mobility before and one week after the operation in all patients. Clinical symptoms and signs of hypocalcemia (numbness of the lips and hands, Chvostek's or Trousseau's sign, carpopedal spasm) and serum calcium concentrations were assessed in patients treated with TT. Hypocalcemia was defined as a postoperative serum calcium level <8 mg/dL. Vocal cord palsy and hypocalcemia were considered permanent when there was no evidence of recovery within 6 months of the operation.
As described in our previous study,1,24 all patients were asked to grade their satisfaction with the cosmetic results and postoperative pain in the neck or anterior chest wall. To compare postoperative functional outcomes between endoscopic and open thyroidectomy 1 and 6 months after surgery, some patients in groups 3 (n=36) and 4 (n=39) without recurrent laryngeal nerve palsy were asked to evaluate their perceptual voice, the voice handicap index (VHI), fatigue during phonation and difficulty with high pitch and singing voice, hypoesthesia or paresthesia in the neck or anterior chest wall, and swallowing difficulty, in a prospective non-randomized manner.
A 5-point visual analogue scale was applied to patients who had independently self-rated their voice function and the degree of discomfort in the neck or anterior chest wall (0=normal; 5=worst). Perceptual voice evaluations were conducted using the grade, roughness, breathiness, aesthenia, strain (GRBAS) rating scale. The five parameters evaluated were overall grade of dysphonia, roughness, breathiness, asthenia, and strain. Professional speech language pathologists evaluated voice quality using the GRBAS scale (0=normal; 1=slight disturbance; 2=moderate disturbance; and 3=severe disturbance). At the same follow-up visit, the patients also completed the VHI, which is a patient-based survey divided into three subscales that measure the functional, physical, and emotional aspects of the handicap caused by voice impairment. The subscale scores range from 0 to 40, and total scores range from 0 to 120; a higher score indicates a greater degree of handicap.
A total of 189 endoscopic thyroidectomized patients were enrolled in this study. In the benign group, group 1 included 95 patients, and group 2 included 37 patients. One-hundred and three patients underwent endoscopic HT, whereas 29 received endoscopic TT. In group 1, we performed 21 TTs and 74 HTs. In group 2, we performed 8 TTs and 29 HTs. All endoscopic procedures were performed successfully with no conversions. The age and gender distributions of the groups were similar (Table 1). Postoperative permanent pathology revealed 30 follicular adenomas, 99 nodular hyperplasias, and 3 cases of lymphocytic thyroiditis (Table 1). In the micropapillary carcinoma group, 57 patients underwent endoscopic HT with ipsilateral CND (group 3) and were compared with 60 patients who received conventional open HT with ipsilateral CND (group 4). The age and gender distributions of the groups were similar (Table 2). Postoperative permanent pathology reconfirmed papillary thyroid carcinomas. In the benign group, the mean tumor size in group 2 (50.92±7.36 mm) was significantly larger than that of group 1 (25.08±8.32 mm; p≤0.001). In the micropapillary carcinoma group, the mean primary tumor size was 7.25±2.30 mm in group 3 (endo group) and 7.10±2.65 mm in group 4 (open group) (Table 2). The difference was not statistically significant (p=0.753). The mean operating time for HT in the benign group was 111.62±37.17 min in group 1 and 113.62±39.37 min in group 2 (p=0.810) (Table 1). The mean operating time for TT in the benign group was 166.43±43.91 min in group 1 and 170.00±45.51 min in group 2 (p=0.848) (Table 1). Overall, the mean operating time for HT was significantly shorter than that of TT in both groups. In the micropapillary carcinoma group, the operating time in group 3 (endo group) was 121.65±20.78 min, which was 50.12 min longer than in group 4 (open group; 71.53±29.43 min) (Table 2), which was statistically significant (p=0.015).
The mean number of dissected central lymph nodes was 4.37±2.74 in group 3 (endo group) and 4.80±2.46 in group 4 (open group) (Table 2), which was not statistically significant (p=0.371). The findings showed central lymph node metastasis in 21 endo group patients (36.8%) and in 26 open group patients (43.3%). The mean number of metastatic central lymph nodes was 1.30±1.83 in group 3 (endo group) and 1.43±1.87 in group 4 (open group) (Table 2); this difference was not statistically significant (p=0.694). Pathologically proven extrathyroidal extension of micropapillary thyroid carcinoma occurred in 10 group 3 patients (endo group; 17.5%) and in 19 group 4 patients (open group; 31.7%). The difference was not statistically significant (p=0.077). A minor injury to the tumor capsule was encountered in some cases in group 2 of the benign group; however, all thyroid tumors were successfully removed without spillage.
The volume and duration of postoperative drainage were similar between the groups (351.68±129.26 mL and 4.51±1.38 days in group 1 and 311.35±86.80 mL and 4.16±1.26 days in group 2, respectively) (Table 1). The postoperative hospital stay was also similar between the two benign groups (5.73±1.22 days in group 1 and 5.22±0.98 days in group 2) (Table 1). However, in the micropapillary carcinoma group, the postoperative hospital stay was 6.21±0.95 days in group 3 (endo group) and 4.42±1.06 days in group 4 (open group) (Table 2), which was statistically significant (p≤0.001).
The recurrent laryngeal nerve was identified in all patients. However, three patients in group 1 (3.2%), two in group 2 (5.4%), two in group 3 (3.5%), and two in group 4 (3.3%) developed transient unilateral vocal cord palsy that resolved spontaneously within 6 months of the operation (Table 3 and 4). When we confirmed the nerve integrity intraoperatively, these patients were followed up without any intervention. Permanent vocal cord palsy occurred in one patient each in groups 1 and 3. No statistically significant difference was observed in terms of vocal cord palsy between the endoscopic and open groups (Table 5).
In terms of preservation of the parathyroid gland, there was no significant difference between the two groups in either the benign or micropapillary carcinomas group. It was generally easier to identify and preserve the superior parathyroid gland than the inferior parathyroid, regardless of study group or operation type (Table 1 and 2). More than one in four parathyroid glands were preserved among the TT cases. Clinical manifestations of hypocalcemia were observed in nine patients in group 1 (42.9%) and in two in group 2 (37.5%), but the frequency of this complication did not differ between groups (p=1.000) (Table 3). All patients with transient postoperative hypocalcemia were discharged on a therapeutic regimen of oral calcium and vitamin D. There were no cases of permanent hypocalcemia in the benign group.
Postoperative hematomas were encountered in three patients (3.16%) in group 1, one (1.8%) in group 3, and one (1.7%) in group 4. The hematomas, which arose from tiny hemorrhagic foci in the subplatysmal flap, were evacuated and controlled without difficulty under local anesthesia. Fifteen patients in group 1 (15.8%), five in group 2 (13.5%), seven (12.3%) in group 3, and five (8.3%) in group 4 developed postoperative seromas, which were resolved by repeated needle aspiration without compressive dressings. No inadvertent injury to adjacent structures, such as the trachea or esophagus, occurred in either group (Table 3 and 4). Excluding cases of seroma, we obtained a relatively similar complication rate in the endoscopic and open groups (Table 5).
Similar to our previous results,1,24 some patients complained of moderate to severe neck or anterior chest wall pain on the first postoperative day. However, postoperative pain decreased thereafter, and few patients complained of moderate or severe pain at 7 days after surgery. Moreover, there was no significant difference in postoperative pain between groups 1 and 2.
As we previously described,1,24 the postoperative cosmetic results were better in group 3 (endo group) than in group 4 (open group), because the small incision scar in the axilla was completely covered when the patient's arm was in a natural position, so the periareolar incision was almost invisible. Most of the subjective parameters (hypoesthesia or paresthesia in the neck or anterior chest wall and difficulty swallowing) improved within 6 months in groups 3 and 4, but difficulty with a high pitched or singing voice didn't improve in group 4 (open group) until 6 months post-operation. VHI also didn't improve until 6 months post-operation in group 3 (endo group) (Fig. 3).
Cosmesis is particularly important to all persons as well as younger women, who constitute a large proportion of patients affected by thyroid diseases. Because the anterior neck is a prominent, constantly exposed part of the body, an unsightly scar can prove very distressing for the patient and for the surgeon.25,26 The pursuit of an esthetically pleasing scar after open thyroid surgery has led surgeons to perform endoscopic surgery on the neck. Since endoscopic thyroid surgery was first successfully performed by Hüscher,1,2 many reports have shown that an endoscopic procedure can be used in selected thyroid nodule cases.2-15,24,27
To our knowledge, there are about 20 published endoscopic thyroidectomy techniques, including some variations.27,28 These can be grouped into those that use small cervical incisions, either ventral or lateral, and those that use the distant chest wall and periareolar, axillary, or combined approaches.1-15,27 All of the approaches have their own merits and drawbacks; however, few reports have described the feasibility of thyroidectomy using an endoscopic approach for large benign thyroid lesions.15,29-32 Furthermore, the use of endoscopic procedures for thyroid cancers remains controversial.
Since we adopted our novel approach for selected patients in 2007, the surgeon's learning curve has gradually stabilized. We speculated that an expanded set of indications will make this procedure available to a broader patient population. We performed a retrospective, observational study of our cohort to document whether expanded indications are feasible for this technique and collected follow-up data regarding the surgical outcomes.
Considering that the most important goal of endoscopic thyroid surgery is to minimize the visible scar in a natural position, incisions on or near the neck, or on the chest wall, which are used in several minimal-access approaches, should be avoided. In fact, cervical approaches can easily cause visible scars on the neck,1,3,15 and anterior chest approaches can cause more hypertrophic scarring or keloids than axillary or circumareolar incisions.4,24 Our unilateral axillo-breast approach is essentially a surgical resection of the thyroid lobe remote from the neck, thus resulting in no neck scar. The access incisions are not necessarily smaller than those in the neck, but they have been shifted to an area that is covered by clothing.
In general, endoscopic thyroidectomy and open thyroid surgery also requires meticulous surgical dissection, absolute hemostasis, en bloc tumor resection, and adequate visualization of the operative field. To accomplish this goal, another important point in the performance of endoscopic thyroid surgery is the convenience of using endoscopic instruments, which can prevent iatrogenic trauma to the thyroid gland and reduce the risk of an incomplete operation or tumor seeding. By applying surgical instruments (endoscopic dissector and HS) at a 45° angle through the axillary and periareolar ports, we achieved a safe and complete thyroidectomy and/or ipsilateral central neck dissection with little difficulty, even in cases of large benign thyroid nodules and micropapillary carcinomas. Because the risk of tumor spillage increases when large thyroid tumors are mobilized in small working spaces with sharp instruments, this technique has been indicated only for a minority of patients. The removal of such tumors may be challenging because of the small working space and the intrinsic difficulty of grasping and manipulating irregularly shaped masses, which may also show a hypervascular capsule. It is also important to avoid iatrogenic trauma to the thyroid capsule, especially in the case of a large goiter, to limit the risk of tumor seeding. In this study, we demonstrated that endoscopic thyroidectomy for tumors ≥4 cm is feasible and safe. Such large thyroid tumors cannot be removed by cervical access or minimally invasive video-assisted thyroidectomy (MIVAT).33-35
With an endoscope to magnify the surgical field and the approach described here, we easily identified and preserved the recurrent laryngeal nerve and parathyroid glands, while performing ipsilateral CND or even among those with tumor diameters ≥4 cm. The superior parathyroid gland was often easier to identify and dissect from the thyroid gland on a vascular pedicle, as in open thyroidectomy. We did not experience any case of permanent hypocalcemia in the TT cases. Although Jeong, et al.20,36 reported a higher incidence of transient hypocalcemia in the endo group than previous reports, we feel that there is little difference between open and endoscopic thyroidectomy in terms of preserving the parathyroid glands. However, functional parathyroid preservation should be validated through a large-scale study.
We speculate that transient vocal cord palsy was related to manipulation of the thyroid gland, postoperative inflammation, edema, thermal injury from the HS, or traction injury during dissection of the recurrent laryngeal nerve. Although nerve integrity was preserved during the operation, we encountered two cases of permanent RLN palsy. Careful dissection with the endoscopic cottonoid and HS is essential while dissecting the thyroid from the trachea to minimize the remnant thyroid tissue near Berry's ligament.
In terms of CND, our experience demonstrates that there was little difference in the numbers of retrieved lymph nodes and safety compared with the open group. However, our approach may have technical limitations and disadvantages especially when complex and difficult procedures are undertaken. Although our approach enables the surgeon to conduct a complete ipsilateral CND from the carotid artery to the substernal notch and the prelaryngeal area, the awkward endoscope angle and manipulation difficulties should be resolved. Also, it is difficult to approach the contralateral upper pole of the thyroid using only our unilateral approach.12,19,21 Some reports, which have described the limitations of a bilateral total thyroidectomy using a one-sided axillary approach, have introduced a bilateral axillary approach.25,37 For the same reason, most of the total thyroidectomy cases in our series were performed with a bilateral axillo-breast approach. Recently, application of the da Vinci S surgical robot system to various surgical fields has led to a speedy evolution in minimally invasive surgical techniques.38-40 Kang, et al.19,36 have applied the da Vinci S surgical robot system to operations on thyroid cancer patients with low risk, using the unilateral axillary approach, and reported that adding the da Vinci S surgical robot system to endoscopic thyroidectomies has resolved the difficulties of conventional endoscopic surgery and provided additional benefits, such as the straightforward identification and preservation of the recurrent laryngeal nerve and parathyroid glands; complete and safe thyroid resection and lymph node dissection in the deep, narrow working space; independence from an assistant; and improved surgeon comfort. They also mentioned that the introduction of the da Vinci robotic system offered the possibility for reducing the limitations of conventional endoscopy.38-40
Before our approach gains wide acceptance for managing various thyroid nodules, including micropapillary carcinoma, our endoscopic thyroidectomy approach has several potential weaknesses in terms of oncologic validity of TT with CND, invasiveness, hospital stay and operating time. First, the debate of a lobectomy versus a total thyroidectomy for well-differentiated thyroid cancer is beyond the scope of this article, as is the issue of selective versus routine clearance of the central compartment lymph node basin (levels VI and VII) in patients with papillary thyroid cancer. However, even for patients with micropapillary thyroid carcinoma with no nodal involvement, total or near total thyroidectomy plus prophylactic CND is advocated.1,41,42 Therefore, total thyroidectomy surgical techniques plus CND via a unilateral or bilateral axillo-breast approach should be verified oncologically. In the present study, we strictly observed the indication of endoscopic thyroidectomy for micropapillary carcinoma, so very few patients with thyroid cancer underwent an endoscopic total thyroidectomy (not included in this study). However, Jeong, et al.20 reported that all total thyroidectomized patients in the endo group had <1 ng/mL of postoperative serum Tg, and, on short-term follow-up, they detected no tumor recurrence in the endo group. Second, our approach is more invasive because of the wide dissection from the axilla to the anterior neck, and is also more time consuming than the conventional open method. Although our approach is superior in terms of cosmetic outcome (i.e., invisible scars in neutral positions), it tends to be more invasive than the cervical approach or MIVAT and requires significant dissection to achieve an adequate working space. Therefore, postoperative pain and functional discomfort were likely concerns. Several patients complained of mild hypoesthesia and paresthesia in the neck and anterior chest wall and discomfort while swallowing, which ceased within 6 to 12 months after the operation, consistent with our previous study.1,24 However, the aspect of invasiveness could be improved regarding some of the subjective parameters (VHI, difficulty with high pitch, and singing voice). To date, we have been unable to find an approach in the literature that is both minimally invasive and totally scarless, although cadaveric investigation has been performed in some institutes, trans-oral thyroidectomy has not yet been applied to patients; thus, our technique represents a valuable alternative to more conventional thyroidectomy methods. Third, a relatively high frequency of seromas likely contributed to the relative increase in length of hospital stay over that of other studies.10,13 The authors expect that seromas resulted from a wide skin flap elevation and creation of a large working space from the axilla to the thyroid gland. Because postoperative seroma could be related to postoperative neck & chest wall adhesion or fibrosis in the healing process, we meticulously detected the formation of postoperative seroma even in small amounts. We speculate that the incidence of seroma was high in our study for these reasons. In our opinion, we should reduce the extent of dissection for prevention of seroma. As we accumulate more experience, the incidence of seroma is falling. Postoperative compressive dressing after removal of drain may be helpful for prevention of seroma. Moreover, in Korea, patients and their families prefer to stay in hospital for longer periods because it is covered under the national health insurance program, and patients often actively participate in decisions regarding the time of hospital discharge. Fourth, the longer operation time than for the open group should be overcome through accumulation of experience or adoption of a robotic-assisted technique. However, as we described previously,24 we experienced a substantial difference in operative time between the first 10 cases and the remaining cases. This reduction in operative time was due to the good surgical view provided by the gasless approach, excellent instrumentation, and the lateral approach to the thyroid gland. Additionally, we ascribe this reduction in operating time to the use of the HS. We used the HS exclusively for hemostasis during endoscopic thyroidectomy. No postoperative hemorrhage or hematoma was noted in the thyroidectomy field using the HS, although a few cases of hematomas arose from the skin flap, elevated with a monopolar coagulator.
Endoscopic thyroidectomy via a gasless axillo-breast approach seems to be a safe procedure even for benign thyroid lesions ≥4 cm and micropapillary carcinomas; it produces outcomes similar to those of open thyroidectomy, requires a longer operative time to be accomplished, and has the advantage of better cosmetic results than open thyroidectomy, although there is room for improvement in terms of invasiveness. Considering that the prevalence of thyroid disease is on the increase these days, ongoing research should focus on developing simultaneously less invasive but cosmetically sufficient endoscopic thyroidectomy techniques that are applicable in patients with large thyroid tumors or micropapillary carcinomas. Longer-term follow-up and prospective studies of the TT and CND surgical techniques are also obviously necessary to address the outcome of patients who are preoperatively demonstrated to have cancer.
Figures and Tables
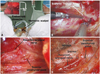 | Fig. 1Axillo-breast approach. (A) A working space is created by inserting an external retractor (Sejong Medical Corporation) through the axillary skin incision. The periareolar skin incision is used for the placement of a 12-mm trocar. (B) The superior thyroid artery (left) is easily identified and sealed off using the harmonic scalpel. (C) The left superior parathyroid gland was identified and preserved between the recurrent laryngeal nerve and common carotid artery (CCA). (D) The left endoscopic hemithyroidectomy was completed. |
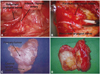 | Fig. 2Surgical procedures for the unilateral axillo-breast approach. (A) The inferior thyroid artery was identified close to the recurrent laryngeal nerve. (B) Hemithyroidectomy with paratracheal lymph node dissection is performed with careful dissection of the recurrent laryngeal nerve. (C) The left hemithyroidectomy-specimen-en bloc with ipsilateral CND is shown. (D) A specimen of large goiter resected via hemithyroidectomy (HT). The surgical specimens showed that thyroidectomy was accomplished without violation of the thyroid capsule. |
 | Fig. 3Postoperative functional outcome [GRBAS scale, Voice Handicap Index (VHI)], easy fatigue during phonation, difficulty with high pitch and singing voice, hypoesthesia or paraesthesia in the neck or anterior chest wall, and swallowing difficulty were analyzed at postoperative months 1 (Post 1M) and 6 (Post 6M). * and ** Significant impairment compared to preoperative period (PreOP) (p<0.05). (A) Subjective parameters-open thyroidectomy group. (B) Subjective parameters-endoscopic thyroidectomy group. |
References
1. Koh YW, Park JH, Kim JW, Lee SW, Choi EC. Endoscopic hemithyroidectomy with prophylactic ipsilateral central neck dissection via an unilateral axillo-breast approach without gas insufflation for unilateral micropapillary thyroid carcinoma: preliminary report. Surg Endosc. 2010. 24:188–197.

2. Hüscher CS, Chiodini S, Napolitano C, Recher A. Endoscopic right thyroid lobectomy. Surg Endosc. 1997. 11:877.

3. Yamashita H, Watanabe S, Koike E, Ohshima A, Uchino S, Kuroki S, et al. Video-assisted thyroid lobectomy through a small wound in the submandibular area. Am J Surg. 2002. 183:286–289.

4. Shimizu K, Akira S, Jasmi AY, Kitamura Y, Kitagawa W, Akasu H, et al. Video-assisted neck surgery: endoscopic resection of thyroid tumors with a very minimal neck wound. J Am Coll Surg. 1999. 188:697–703.

5. Park YL, Han WK, Bae WG. 100 cases of endoscopic thyroidectomy: breast approach. Surg Laparosc Endosc Percutan Tech. 2003. 13:20–25.
6. Ohgami M, Ishii S, Arisawa Y, Ohmori T, Noga K, Furukawa T, et al. Scarless endoscopic thyroidectomy: breast approach for better cosmesis. Surg Laparosc Endosc Percutan Tech. 2000. 10:1–4.

7. Jung EJ, Park ST, Ha WS, Choi SK, Hong SC, Lee YJ, et al. Endoscopic thyroidectomy using a gasless axillary approach. J Laparoendosc Adv Surg Tech A. 2007. 17:21–25.

8. Ikeda Y, Takami H, Sasaki Y, Takayama J, Niimi M, Kan S. Comparative study of thyroidectomies. Endoscopic surgery versus conventional open surgery. Surg Endosc. 2002. 16:1741–1745.
9. Kim JS, Kim KH, Ahn CH, Jeon HM, Kim EG, Jeon CS. A clinical analysis of gasless endoscopic thyroidectomy. Surg Laparosc Endosc Percutan Tech. 2001. 11:268–272.

10. Shimazu K, Shiba E, Tamaki Y, Takiguchi S, Taniguchi E, Ohashi S, et al. Endoscopic thyroid surgery through the axillo-bilateral-breast approach. Surg Laparosc Endosc Percutan Tech. 2003. 13:196–201.

11. Yeh TS, Jan YY, Hsu BR, Chen KW, Chen MF. Video-assisted endoscopic thyroidectomy. Am J Surg. 2000. 180:82–85.

12. Yoon JH, Park CH, Chung WY. Gasless endoscopic thyroidectomy via an axillary approach: experience of 30 cases. Surg Laparosc Endosc Percutan Tech. 2006. 16:226–231.

13. Chung YS, Choe JH, Kang KH, Kim SW, Chung KW, Park KS, et al. Endoscopic thyroidectomy for thyroid malignancies: comparison with conventional open thyroidectomy. World J Surg. 2007. 31:2302–2306.

14. Choe JH, Kim SW, Chung KW, Park KS, Han W, Noh DY, et al. Endoscopic thyroidectomy using a new bilateral axillo-breast approach. World J Surg. 2007. 31:601–606.

15. Bellantone R, Lombardi CP, Bossola M, Boscherini M, De Crea C, Alesina PF, et al. Video-assisted vs conventional thyroid lobectomy: a randomized trial. Arch Surg. 2002. 137:301–304.
16. Henry JF, Raffaelli M, Iacobone M, Volot F. Video-assisted parathyroidectomy via the lateral approach vs conventional surgery in the treatment of sporadic primary hyperparathyroidism: results of a case-control study. Surg Endosc. 2001. 15:1116–1119.

17. Sakuraba M, Miyamoto H, Oh S, Shiomi K, Sonobe S, Takahashi N, et al. Video-assisted thoracoscopic lobectomy vs. conventional lobectomy via open thoracotomy in patients with clinical stage IA non-small cell lung carcinoma. Interact Cardiovasc Thorac Surg. 2007. 6:614–617.

18. Tschernko EM, Hofer S, Bieglmayer C, Wisser W, Haider W. Early postoperative stress: video-assisted wedge resection/lobectomy vs. conventional axillary thoracotomy. Chest. 1996. 109:1636–1642.
19. Kang SW, Jeong JJ, Yun JS, Sung TY, Lee SC, Lee YS, et al. Robot-assisted endoscopic surgery for thyroid cancer: experience with the first 100 patients. Surg Endosc. 2009. 23:2399–2406.

20. Jeong JJ, Kang SW, Yun JS, Sung TY, Lee SC, Lee YS, et al. Comparative study of endoscopic thyroidectomy versus conventional open thyroidectomy in papillary thyroid microcarcinoma (PTMC) patients. J Surg Oncol. 2009. 100:477–480.

21. Ikeda Y, Takami H, Sasaki Y, Kan S, Niimi M. Endoscopic neck surgery by the axillary approach. J Am Coll Surg. 2000. 191:336–340.
22. Gagner M, Inabnet WB 3rd. Endoscopic thyroidectomy for solitary thyroid nodules. Thyroid. 2001. 11:161–163.

23. Colonna M, Guizard AV, Schvartz C, Velten M, Raverdy N, Molinie F, et al. A time trend analysis of papillary and follicular cancers as a function of tumour size: a study of data from six cancer registries in France (1983-2000). Eur J Cancer. 2007. 43:891–900.

24. Koh YW, Kim JW, Lee SW, Choi EC. Endoscopic thyroidectomy via a unilateral axillo-breast approach without gas insufflation for unilateral benign thyroid lesions. Surg Endosc. 2009. 23:2053–2060.

25. Miyano G, Lobe TE, Wright SK. Bilateral transaxillary endoscopic total thyroidectomy. J Pediatr Surg. 2008. 43:299–303.

26. Rafferty M, Miller I, Timon C. Minimal incision for open thyroidectomy. Otolaryngol Head Neck Surg. 2006. 135:295–298.

27. Schardey HM, Schopf S, Kammal M, Barone M, Rudert W, Hernandez-Richter T, et al. Invisible scar endoscopic thyroidectomy by the dorsal approach: experimental development of a new technique with human cadavers and preliminary clinical results. Surg Endosc. 2008. 22:813–820.

28. Yeung GH. Endoscopic thyroid surgery today: a diversity of surgical strategies. Thyroid. 2002. 12:703–706.

29. Slotema ET, Sebag F, Henry JF. What is the evidence for endoscopic thyroidectomy in the management of benign thyroid disease? World J Surg. 2008. 32:1325–1332.

30. Inabnet WB 3rd, Jacob BP, Gagner M. Minimally invasive endoscopic thyroidectomy by a cervical approach. Surg Endosc. 2003. 17:1808–1811.

31. Duncan TD, Rashid QN, Speights F. Surgical excision of large multinodular goiter using an endoscopic transaxillary approach: a case report. Surg Laparosc Endosc Percutan Tech. 2008. 18:530–535.

32. Ruggieri M, Straniero A, Genderini M, D'Armiento M, Fumarola A, Trimboli P, et al. The size criteria in minimally invasive video-assisted thyroidectomy. BMC Surg. 2007. 7:2.

33. Miccoli P, Minuto MN, Ugolini C, Pisano R, Fosso A, Berti P. Minimally invasive video-assisted thyroidectomy for benign thyroid disease: an evidence-based review. World J Surg. 2008. 32:1333–1340.

34. Miccoli P, Berti P, Raffaelli M, Conte M, Materazzi G, Galleri D. Minimally invasive video-assisted thyroidectomy. Am J Surg. 2001. 181:567–570.

35. Miccoli P, Berti P, Raffaelli M, Materazzi G, Baldacci S, Rossi G. Comparison between minimally invasive video-assisted thyroidectomy and conventional thyroidectomy: a prospective randomized study. Surgery. 2001. 130:1039–1043.

36. Kang SW, Lee SC, Lee SH, Lee KY, Jeong JJ, Lee YS, et al. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery. 2009. 146:1048–1055.

37. Lobe TE, Wright SK, Irish MS. Novel uses of surgical robotics in head and neck surgery. J Laparoendosc Adv Surg Tech A. 2005. 15:647–652.

38. Gutt CN, Oniu T, Mehrabi A, Kashfi A, Schemmer P, Büchler MW. Robot-assisted abdominal surgery. Br J Surg. 2004. 91:1390–1397.

39. Savitt MA, Gao G, Furnary AP, Swanson J, Gately HL, Handy JR. Application of robotic-assisted techniques to the surgical evaluation and treatment of the anterior mediastinum. Ann Thorac Surg. 2005. 79:450–455.

40. Link RE, Bhayani SB, Kavoussi LR. A prospective comparison of robotic and laparoscopic pyeloplasty. Ann Surg. 2006. 243:486–491.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download