Abstract
Purpose
Laparoscopic colectomy has clinical benefits such as short hospital stay, less postoperative pain, and early return of bowel function. However, objective evidence of its immunologic and oncologic benefits is scarce. We compared functional recovery after open versus laparoscopic sigmoidectomy and investigated the effect of open versus laparoscopic surgery on acute inflammation as well as tumor stimulation.
Materials and Methods
A total of 57 patients who were diagnosed with sigmoid colon cancer were randomized for elective conventional or laparoscopically assisted sigmoidectomy. Serum samples were obtained preoperatively and on postoperative day 1. C-reactive protein (CRP) and interleukin-6 (IL-6) were measured as inflammation markers, and vascular endothelial growth factor (VEGF) and insulin-like growth factor binding protein-3 (IGFBP-3) were used as tumor stimulation factors. Clinical parameters and serum markers were compared.
Results
Postoperative hospital stay (p=0.031), the first day of gas out (p=0.016), and the first day of soft diet (p<0.001) were significantly shorter for the laparoscopic surgery group than the open surgery group. The levels of CRP, IL-6, and VEGF rose significantly, and the concentration of IGFBP-3 fell significantly after both open and laparoscopic surgery. However, there were no significant differences in the preoperative and postoperative levels of CRP, IL-6, VEGF, and IGFBP-3 between the two groups.
Since the first report of a laparoscopic colectomy by Jacobs, et al.1 in 1991, several prospective studies have reported that laparoscopic surgery was similar to conventional open surgery in terms of oncologic safety.2-4 Moreover, laparoscopic colectomy has benefits like shorter hospital stay, less postoperative pain, and early return of bowel function.5 These favorable early outcomes of laparoscopic colectomy have been shown in a number of studies.6-8 As a result, laparoscopic colectomy has been widely accepted for resectable carcinoma. However, these early outcomes of laparoscopic surgery are based on clinical observations. Some investigators have compared patterns of postoperative cytokine and stress responses with laparoscopically assisted colectomy to conventional open colectomy.9-14 C-reactive protein (CRP), interleukin (IL)-1, IL-6, and tumor necrosis factor-α (TNF-α) have been used as major acute-phase response mediators.15 However, results of these studies have been proved inconsistent. This inconsistency has been attributed to inhomogenous operation type, different sampling timing, and enrollment of immunocompromized patients. Therefore, the impact of laparoscopic colectomy for carcinoma on acute inflammation has not yet been established.
Recently, Belizon, et al.16 reported that vascular endothelial growth factor (VEGF) which is an important inducer of angiogenesis was significantly higher in open surgery than in laparoscopic surgery. Angiogenesis plays a critical role in the growth and development of tumor.17 Therefore, a high level of VEGF may influence the growth of tumor.18 It is possible that open surgery stimulates residual tumor cells more than laparoscopic surgery does.
Another tumor stimulation factor whose level changes after surgery and may consequently impact tumor growth is insulin-like growth factor binding protein-3 (IGFBP-3). Matrix metalloproteinase 9 (MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) may also impact tumor growth.19 Kirman, et al.20 demonstrated that postoperative plasma with a low level of IGFBP-3 withdrawn from patients who had undergone major operation stimulated tumor growth in vitro compared with preoperative plasma. This postoperative reduction of IGFBP-3 was shown to be more dramatic in open surgery than in laparoscopic surgery.21 Although it is not clinically proven that a high level of VEGF and a low level of IGFBP-3 may have an impact on tumor growth, open surgery has stronger association with tumor stimulation factors than in laparoscopic surgery does. Interestingly, inflammatory cytokines, including IL-6, have been reported to be associated with tumor stimulating factors such as VEGF and IGFBP-3.22,23
However, few studies have compared both acute inflammation and tumor stimulating factors in homogenous group of patients who were undergoing open and laparoscopic surgery. In the current study, we compared functional recovery after open versus laparoscopic surgery for sigmoid colon cancer, and investigated the effect of open versus laparoscopic surgery on acute inflammation as well as tumor stimulation. In addition, the relationship between the postoperative elevation of IL-6 and changes of VEGF and IGFBP-3 was examined, on the proposition that this alteration might have effect on the tumor growth.
We included 81 patients who were diagnosed of having resectable sigmoid colon cancer from October 2008 to June 2009. Patients were randomized for elective conventional or laparoscopically assisted sigmoidectomy using random numbers. Preoperative evaluations consisted of history taking, physical examination, carcinoembryonic antigen (CEA) level, peripheral blood test, colonoscopy, and computed tomography. Exclusion criteria were as follows: distant metastasis, blood transfusion, immunosuppressant medication, and history of chemotherapy or radiotherapy treatment. Informed consent was obtained from each patient. This study was approved by the Institutional Review Board.
All patients were treated with premedication and had standard anesthesia. The operations were performed by experienced surgeon (NK Kim) in both open and laparoscopically assisted technique. All open surgeries were performed through a midline skin incision. After mobilizing the mesocolon, the inferior mesenteric artery and vein were doubly ligated and divided. Anastomosis was accomplished using a circular stapler after sigmoidectomy.
In the laparoscopically assisted sigmoidectomy, patient's position of operating room was a modified lithotomy with right side and head down. An initial 12-mm camera port was inserted using the open technique, and pneumoperitoneum was accomplished with carbon dioxide. Two 5-mm ports were introduced into the upper right and left abdominal quadrants and two more 12-mm ports were inserted in the lower right and left abdominal quadrants under laparoscopic visual guidance. After mobilizing the mesocolon, the inferior mesenteric artery and vein were ligated with laparoscopic clips and divided with laparoscopic scissors. The specimen was extracted from mini-laparotomy incision in the left lower abdominal quadrant after transection of distal bowel and extracorporeal preparation for end-to-end anastomosis was completed after proximal bowel resection. Intracorporeal anastomosis was achieved using a circular stapler and the wound was closed with absorbable sutures. In both surgical techniques, a Jackson-Pratt drain was placed in the pelvis for drainage.
After both open and laparoscopic surgeries, a clear liquid diet was started one day after patients passed flatus. Patients were given 1 mg/kg of pethidine every six hours until the first postoperative day (POD1) or upon their request after surgery. Patients were discharged if following discharge criteria were met: 1) passing stool, 2) tolerating soft diet, 3) being comfortable on oral analgesia, 4) white blood cell count within the normal range, and 5) being happy to be discharged with full ambulation.
After surgery, we collected their clinical data, including operation time, amount of transfusion, time of the first passing flatus, time when soft diet began, duration of postoperative hospital stay, complications, and pathologic report.
Blood was taken before surgery and the POD1. Seven milliliters of blood was withdrawn from all patients by peripheral venipuncture. The specimens were centrifuged within four hours after withdrawal. The serum was collected and stored at -70℃ until enzyme-linked immunosorbent assay (ELISA) was performed. CRP and IL-6 were measured as inflammation markers, and VEGF and IGFBP-3 were used as tumor stimulation factors. CRP, IL-6, VEGF, and IGFBP-3 level were determined in duplicate using ELISA test (Millipore, Billerica, MA, USA for CRP; BD Biosciences, San Jose, CA, USA for IL-6; RayBiotech, Inc., Norcross, GA, USA for IGFBP-3 and VEGF). The results of ELISA were read using an automated microplate reader (SpectraMax Plus384, Sunnyvale, CA, USA) and calculated with software program (SoftMax Pro, Sunnyvale, CA, USA).
On the basis of our institute's data base over the last five years, the mean difference of hospital stay between open and laparoscopically assisted sigmoidectomies was 4.53 days. Assuming a pooled standard deviation of 4.8 days, and assigning α=0.05, β=0.15, 10% of dropout rate, and 1 : 2 allocation, at least 57 patients were needed. We decided to apply 1 : 2 allocation of recruits because patients preferred to receive laparoscopic surgery.
Statistical analysis was carried out using SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA). Mann-Whitney test for continuous variables and Fisher's exact tests for categorical variables were used for statistical comparison of clinical characteristics. Difference within each group was tested with the Wilcoxon matched pairs test. A Spearman correlation coefficient was determined to correlate changes in VEGF and IGFBP-3 levels with postoperative IL-6 levels, respectively. A value of p<0.05 was considered significant.
A total of 81 patients were recruited in this study. Nine patients with immunosuppressant medication, distant metastasis, and transfusion were excluded. Two patients had anastomotic leakage, and one patient had tumor perforation at the time of surgery. Twelve patients were excluded because their samples were found to be inappropriate for ELISA test during the collection process. As a result, we enrolled 57 patients in the study. A flow chart of patient selection is described in Fig. 1. Nineteen patients received open surgery, and 38 patients received laparoscopic surgery. The two groups were comparable in terms of age, gender, body mass index, preoperative peripheral neutrophil count, preoperative CEA level, preoperative morbidity, and the American Society of Anesthesiologists score. However, the mean operation time was significantly longer in the laparosopic surgery group, whereas postoperative hospital stay, the first day of gas out, and the first day of soft diet were significantly shorter and earlier in the laparoscopic surgery group compared to the open surgery group. A summary of patient characteristics comparing open and laparoscopic surgeries is shown in Table 1.
No differences in serum concentrations of preoperative and postoperative CRP, IL-6, IGFBP-3, and VEGF were observed between the two groups (Table 2). However, CRP, IL-6, and VEGF levels rose significantly after both open and laparoscopic surgeries (p<0.001 in each group) (Figs. 2, 3 and 4). In contrast, the concentration of IGFBP-3 decreased significantly after open and laparoscopic surgeries (p=0.03 in the open surgery group and p=0.003 for the laparoscopic surgery group) (Fig. 5). However, there were no significant differences in CRP, IL-6, VEGF, and IGFBP-3 levels between the open and laparoscopic surgery groups.
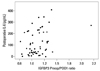
There was a significant correlation between the POD1 IL-6 level and a drop in VEGF which was calculated as a ratio between POD1/preoperative VEGF (r=0.293; p=0.027) (Fig. 6). In addition, a drop in IGFBP-3 calculated as a ratio between preoperative/POD1 IGFBP-3 also had significant correlation with POD1 IL-6 (r=0.354; p=0.007) (Fig. 7).
In several prospective studies, laparoscopic colectomy has been shown to have oncologic outcomes similar to those of open colectomy.2-4 Laparoscopic surgery is also known to be associated with short hospital stay, less postoperative pain, and early resumption of normal diet.5 This favorable early outcome has been regarded to be related to reduced bowel injury, surgical metabolic stress, and immunosuppressive response.24 CRP is a key marker of acute-phase proteins as its level increases in proportion to the degree of inflammation. Plasma concentration of CRP serves as a reliable screening test for acute-phase response. The CRP level usually rises 4 to 12 hours after surgery and peak at 24 hours. An elevated CRP level may remain for approximately two weeks.25 The acute-phase response consists of a series of hormonal, metabolic, and immunologic changes in response to surgery, trauma, or sepsis.26 TNF-α, IL-1β, and IL-6 are major components of the acute-phase response in humans. Among these mediators, IL-6 is primarily responsible for the hepatic component of the response, resulting in the synthesis of acute-phase proteins. IL-6 usually peaks at 4 to 48 hours after surgery and rapidly falls afterward. The levels of the cytokines and CRP have been identified to correspond with the severity of surgery and the presence of complications. Thus, these markers have been used to reflect surgical tissue trauma.26
Previous studies on acute-phase response after laparoscopic colorectal surgery have been inconclusive.9-14 Leung, et al.9 and Delgado, et al.14 demonstrated that an acute-phase response was less in the laparoscopic surgery group than in the open surgery group. Therefore, they suggested that the difference in the systemic cytokine response may affect anti-inflammation and immunosuppression. In contrast, however, Tang, et al.10 Fukushima, et al.12 and Dunker, et al.13 described no differences in the acute-phase response in patients receiving conventional open surgery and those who had laparoscopic surgery for colorectal cancer. Interestingly, Stage, et al.11 showed that CRP and IL-6 peak levels were higher in the laparoscopic than in the open group. These studies were relatively uniform and conducted with randomized fashion. However, some of these studies included metastatic disease, immunocompromized patients, and inconstant surgical procedure. In the present study, we used a homogenous group of patients with sigmoid colon cancer, and excluded patients with metastatic disease, use of immunosuppressant, and intraoperative transfusion.
In our study, CRP and IL-6 levels increased significantly after both open and laparoscopic sigmoidectomy (p<0.001) (Figs. 2 and 3), indicating significant activation of inflammatory response. There were no significant differences in the postoperative CRP and IL-6 levels between the open and laparoscopic surgery groups. This phenomenon could be explained by surgical bowel injury, which was comparable in both surgical techniques. The IL-6 level after laparoscopic surgery may vary greatly because of surgical technique and patient selection. Technique of laparoscopic surgery is influenced by surgeon's experience, patient factor, and tumor factor. In contrast, open surgery is well established and give similar results. According to our data, the level of postoperative IL-6 level was more variable in the laparoscopic than in the surgery group. There was a problem in the current study that deserves to be mentioned. Time interval from the end of surgery and sampling time of the first postoperative day varied in each patient. Therefore, our study was limited by the bias inherent to quantitative analysis of this nature.
The early outcomes of the laparoscopic surgery group were remarkable. There were significant differences in operation time, postoperative hospital stay, and return of bowel function between open and laparoscopic surgery groups. Clinical outcomes after laparoscopic surgery were better than after open surgery, although laparoscopic surgery group needed longer operation time.
A recent study evaluated plasma VEGF levels preoperatively and on days 1 and 3 after open and laparoscopic colorectal resections for benign and malignant diseases as well as after gastric bypass for morbid obesity.16 This study demonstrated that the mean values of VEGF significantly increased on POD 1 and 3 after colorectal resection and gastric bypass, and that the extent of elevation at postoperative time was significantly greater for the open surgery group. An elevated VEGF level may remain unchanged for approximately three weeks after curative surgery.18
A high level of VEGF may stimulate the development and growth of metastases.27 Major surgery has been associated with IGFBP-3 depletion and increased plasma MMP-9 levels, which are related with tumor stimulation.19,20 In our study, the VEGF level increased and the IGFBP-3 level decreased significantly after both open and laparoscopic sigmoidectomies (p<0.001 for VEGF in both open and laparoscopic surgery; p=0.03 for IGFBP-3 in open surgery, p=0.003 for IGFBP-3 in laparoscopic surgery) (Figs. 4 and 5). However, there were no significant differences in the postoperative VEGF and IGFBP-3 levels between the open and laparoscopic surgery groups. Do increased VEGF and decreased IGFBP-3 levels after both open and laparoscopic surgeries affect tumor growth? Should clinicians start anti-tumor chemotherapy in early postoperative time? However, it is not clear whether such therapy would be safe or effective in early time of post-operation. Moreover, plasma VEGF levels did not increase in all patients after surgery and varied broadly from patient to patient.18 Therefore, clinical importance of these findings remains largely unclear.
In the present study, we demonstrated that a rise in the VEGF concentration and reduction in the IGFBP-3 concentration after surgery were associated with the postoperative IL-6 level. Similarly, Kirman, et al.23 showed that the IL-6 level on POD1 was found to be significantly correlated with a decrease in plasma IGFBP-3 after open surgery (r=0.81, p<0.001). However, it is not known how exactly IL-6 affects VEGF and IGFBP-3 balance. Several investigators have reported that IL-6 induced gene expression of various protease such as cathepsins and metalloproteinase-13, and enhanced IGFBP-3 proteolysis.28-30 Moreover, IL-6 has been found to upregulate VEGF expression using various molecular pathways.22 However, our results suggest that an acute inflammatory response may be correlated with VEGF and IGFBP-3 which are associated with tumor stimulation. Knowledge on the inflammatory response-mediated pathway of VEGF production and IGFBP-3 depletion may result in novel therapeutic strategies for colon cancer patients undergoing surgery. The relationship of inflammation and tumor stimulation after surgery should be taken into consideration and requires further evaluation in the future.
In conclusion, we demonstrated herein that early outcome after laparoscopic surgery was better than that after open surgery despite longer operation time. Thus, laparoscopic sigmoidectomy is an acceptable and safe procedure for sigmoid colon cancer. This study did not find any difference in preoperative and postoperative CRP, IL-6, VEGF, and IGFBP-3 levels between the open and laparoscopic surgery groups. However, the levels of these markers were significantly changed after both open and laparoscopic surgeries. Postoperative increase of the VEGF level and decrease of IGFBP-3 level were correlated with the postoperative IL-6 level. Inflammation markers and tumor stimulating factors may not reflect clinical benefit of laparoscopic surgery.
Figures and Tables
Fig. 2
Changes in the serum concentration of C-reactive protein (CRP) in patients undergoing open or laparoscopic surgery for sigmoid colon cancer. No significant differences were found between the open and laparoscopic surgery groups. *p<0.001 Preoperative (Preop) value versus the first postoperative day (POD1).

Fig. 3
Changes in the serum concentration of interleukin-6 (IL-6) in patients undergoing open or laparoscopic surgery for sigmoid colon cancer. No significant differences were found between the open and laparoscopic surgery groups. *p<0.001 Preoperative (Preop) value versus the first postoperative day (POD1).

Fig. 4
Changes in the serum concentration of vascular endothelial growth factor (VEGF) in patients undergoing open or laparoscopic surgery for sigmoid colon cancer. No significant differences were found between the open and laparoscopic surgery groups. *p<0.001 Preoperative (Preop) value versus the first postoperative day (POD1).

Fig. 5
Changes in the serum concentration of insulin-like growth factor binding protein-3 (IGFBP-3) in patients undergoing open or laparoscopic surgery for sigmoid colon cancer. No significant differences were found between the open and laparoscopic surgery groups. *p=0.03 Preoperative (Preop) value versus the first postoperative day (POD1). †p=0.003 Preoperative (Preop) value versus the first postoperative day (POD1).

Fig. 6
A postoperative increase of vascular endothelial growth factor (VEGF) correlates with a concentration of the first postoperative day (POD1) interleukin-6 (r=0.293, p=0.027). The ratio between the values of the POD1 versus preoperative (Preop) VEGF was used to analyze for a postoperative increase of concentration.

Fig. 7
A postoperative decrease of insulin-like growth factor binding protein-3 (IGFBP-3) correlated with a concentration of the first postoperative day (POD1) interleukin-6 (r=0.354, p=0.007). The ratio between the values of preoperative (Preop) versus the POD1 IGFBP-3 was used to analyze for a postoperative loss of the protein.
ACKNOWLEDGEMENTS
This study was supported by grants of the Korea Healthcare technology R&D project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A040001, A040151).
References
1. Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc. 1991. 1:144–150.
2. Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004. 363:1187–1192.

3. Scheidbach H, Schneider C, Hügel O, Scheuerlein H, Bärlehner E, Konradt J, et al. Oncological quality and preliminary long-term results in laparoscopic colorectal surgery. Surg Endosc. 2003. 17:903–910.

4. Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002. 359:2224–2229.

5. Yun SS, Hwang DW, Kim SW, Park SH, Park SJ, Lee DS, et al. Better treatment strategies for patients with acute cholecystitis and American Society of Anesthesiologists classification 3 or greater. Yonsei Med J. 2010. 51:540–545.

6. Kwok SP, Lau WY, Carey PD, Kelly SB, Leung KL, Li AK. Prospective evaluation of laparoscopic-assisted large bowel excision for cancer. Ann Surg. 1996. 223:170–176.

7. Reza MM, Blasco JA, Andradas E, Cantero R, Mayol J. Systematic review of laparoscopic versus open surgery for colorectal cancer. Br J Surg. 2006. 93:921–928.

8. Tjandra JJ, Chan MK. Systematic review on the short-term outcome of laparoscopic resection for colon and rectosigmoid cancer. Colorectal Dis. 2006. 8:375–388.

9. Leung KL, Lai PB, Ho RL, Meng WC, Yiu RY, Lee JF, et al. Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma: a prospective randomized trial. Ann Surg. 2000. 231:506–511.

10. Tang CL, Eu KW, Tai BC, Soh JG, MacHin D, Seow-Choen F. Randomized clinical trial of the effect of open versus laparoscopically assisted colectomy on systemic immunity in patients with colorectal cancer. Br J Surg. 2001. 88:801–807.

11. Stage JG, Schulze S, Møller P, Overgaard H, Andersen M, Rebsdorf-Pedersen VB, et al. Prospective randomized study of laparoscopic versus open colonic resection for adenocarcinoma. Br J Surg. 1997. 84:391–396.

12. Fukushima R, Kawamura YJ, Saito H, Saito Y, Hashiguchi Y, Sawada T, et al. Interleukin-6 and stress hormone responses after uncomplicated gasless laparoscopic-assisted and open sigmoid colectomy. Dis Colon Rectum. 1996. 39:S29–S34.

13. Dunker MS, Ten Hove T, Bemelman WA, Slors JF, Gouma DJ, Van Deventer SJ. Interleukin-6, C-reactive protein, and expression of human leukocyte antigen-DR on peripheral blood mononuclear cells in patients after laparoscopic vs. conventional bowel resection: a randomized study. Dis Colon Rectum. 2003. 46:1238–1244.

14. Delgado S, Lacy AM, Filella X, Castells A, García-Valdecasas JC, Pique JM. Acute phase response in laparoscopic and open colectomy in colon cancer: randomized study. Dis Colon Rectum. 2001. 44:638–646.

15. Vittimberga FJ Jr, Foley DP, Meyers WC, Callery MP. Laparoscopic surgery and the systemic immune response. Ann Surg. 1998. 227:326–334.

16. Belizon A, Balik E, Feingold DL, Bessler M, Arnell TD, Forde KA, et al. Major abdominal surgery increases plasma levels of vascular endothelial growth factor: open more so than minimally invasive methods. Ann Surg. 2006. 244:792–798.

17. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990. 82:4–6.

18. Belizon A, Balik E, Horst P, Feingold D, Arnell T, Azarani T, et al. Persistent elevation of plasma vascular endothelial growth factor levels during the first month after minimally invasive colorectal resection. Surg Endosc. 2008. 22:287–297.

19. Kirman I, Jain S, Cekic V, Belizon A, Balik E, Sylla P, et al. Altered plasma matrix metalloproteinase-9/tissue inhibitor of matrix [corrected] metalloproteinase-1 concentration during the early postoperative period in patients with colorectal cancer. Surg Endosc. 2006. 20:482–486.

20. Kirman I, Cekic V, Poltaratskaia N, Asi Z, Bessler M, Huang EH, et al. Plasma from patients undergoing major open surgery stimulates in vitro tumor growth: lower insulin-like growth factor binding protein 3 levels may, in part, account for this change. Surgery. 2002. 132:186–192.

21. Kirman I, Cekic V, Poltoratskaia N, Sylla P, Jain S, Forde KA, et al. Open surgery induces a dramatic decrease in circulating intact IGFBP-3 in patients with colorectal cancer not seen with laparoscopic surgery. Surg Endosc. 2005. 19:55–59.

22. Angelo LS, Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin Cancer Res. 2007. 13:2825–2830.

23. Kirman I, Poltaratskaia N, Cekic V, Forde KA, Ansari P, Boulay C, et al. Depletion of circulating insulin-like growth factor binding protein 3 after open surgery is associated with high interleukin-6 levels. Dis Colon Rectum. 2004. 47:911–917.

24. Maruszynski M, Pojda Z. Interleukin 6 (IL-6) levels in the monitoring of surgical trauma. A comparison of serum IL-6 concentrations in patients treated by cholecystectomy via laparotomy or laparoscopy. Surg Endosc. 1995. 9:882–885.
25. Ohzato H, Yoshizaki K, Nishimoto N, Ogata A, Tagoh H, Monden M, et al. Interleukin-6 as a new indicator of inflammatory status: detection of serum levels of interleukin-6 and C-reactive protein after surgery. Surgery. 1992. 111:201–209.
26. Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ. Systemic cytokine response after major surgery. Br J Surg. 1992. 79:757–760.

27. Jung YD, Nakano K, Liu W, Gallick GE, Ellis LM. Extracellular signal-regulated kinase activation is required for up-regulation of vascular endothelial growth factor by serum starvation in human colon carcinoma cells. Cancer Res. 1999. 59:4804–4807.
28. Gerber A, Wille A, Welte T, Ansorge S, Buhling F. Interleukin-6 and transforming growth factor-beta 1 control expression of cathepsins B and L in human lung epithelial cells. J Interferon Cytokine Res. 2001. 21:11–19.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download