INTRODUCTION
Teicoplanin, one of the glycopeptide antibiotics, is primarily active against Gram-positive microorganisms including Staphylococcus, Streptococcus, and Enterococcus species. During chemotherapy for hematologic malignancies, Gram-positive bacterial infections are a common cause of neutropenic fever in Korea as well as in western countries.
1,
2
Teicoplanin is known to have bi- to tri-exponential distribution features and a longer elimination half-life than vancomycin, which makes its once daily administration possible. It is also generally accepted that teicoplanin with a trough concentration (C
trough) >10 mg/L is clinically effective; to ensure this trough concentration, a dose regimen of 6 mg/kg (400 mg) every 12 hours for 3 doses, then daily, are the minimal requirements for all patients with normal renal function, and a C
trough of 20 mg/L should be exceeded in cases of deep-seated staphylococcal infections.
3,
4 Moreover, according to Harding, et al.,
5 the mean trough concentration was correlated with the clinical outcome of patients with
Staphylococcus aureus septicemia. MacGowan, et al.
6 also demonstrated that the C
trough >10 mg/L and C
trough >20 mg/L of teicoplanin were related to favorable outcomes and curing of staphylococcal infections, respectively.
Dose regimens based upon trough concentrations have not considered the microorganism-specific factors. For the appropriate use of an antimicrobial agent, physicians should be aware of microorganism-specific as well as patient-specific factors. To do this, a clear relationship between pharmacokinetic (PK) and pharmacodynamic (PD) interaction of antimicrobial agents must be elucidated. The minimum inhibitory concentration (MIC) is one of the microorganism related factors that requires consideration when determining treatment doses and durations for the best clinical outcome.
7 In the case of glycopeptides, the AUC
24 (area under the drug concentration-time curve during 24 hr) over the MIC i.e., AUC
24/MIC that associates drug exposure with the characteristics of the microorganism has recently come into use as a surrogate marker of clinical efficacy. However, there has been some debate as to the optimal level of this target for treatment using teicoplanin. Although the AUC
24/MIC >125 has been regarded as a PK-PD marker that can predict clinical success with vancomycin treatment,
7,
8 a level of AUC
24/MIC >345 have recently been recommended as the target to be attained.
9,
10 However, the AUC
24/MIC target values for teicoplanin treatment have not been extensively studied. Therefore, clinicians have adopted those from vancomycin with the rationale that both antibiotics belong to the same glycopeptide class.
In this context, the present study was performed to assess the target attainment rate (TAR) for microorganism-nonspecific (Ctrough) and microorganism-specific (AUC24/MIC) targets during two weeks of teicoplanin administration for the treatment of S. aureus strains in Korean patients with neutropenic fever under several dose regimens.
DISCUSSION
According to a recent meta-analysis of 24 randomized controlled trials that compared vancomycin to teicoplanin, there were no significant differences between teicoplanin and vancomycin in terms of efficiency outcomes, such as clinical and microbiological failure rate; however, teicoplanin was associated with a lower occurrence of adverse events than vancomycin.
13
Although teicoplanin has been widely used for infections associated with hematological malignancies in Korea, due to its low rate of adverse drug reactions, a population-specific optimum dose regimen has not been established with respect to its association with the PK/PD. Therefore, the goal of this study was to determine the appropriate teicoplanin-dosing strategy for adequate treatment of S. aureus in Korean patients with neutropenic fever.
To determine the effective dose regimen, validated endpoints or surrogate markers for clinical success or failure are required. In the case of teicoplanin, the trough concentration and AUC24/MIC are considered as microorganism-nonspecific and microorganism-specific markers, respectively. Moreover, the time to achieve a target value for the above marker must also be considered to associate the above markers with the clinical outcome. Because the loading dose could be readily determined from the maintenance dose using PK principles, for simplicity, the TAR with regard to the maintenance dose was studied. Both the trough concentration and AUC24/MIC are associated with the PK properties of a specific population, i.e., patients with neutropenic fever that have hematological malignancies. While the trough concentration is directly related to the dose regimen and the patient pharmacokinetics, for the AUC24/MIC, the MIC value for the microorganism has to be considered as well.
In a study reported by Whitehouse, et al.
14 on teicoplanin PK in critically ill patients with renal impairment, the trough concentrations were not significantly different in the patients that were cured (mean 5.2-8.7 mg/L) and in those that failed to respond to treatment (mean 9.3-12.1 mg/L). According to Gimenez, et al.,
15 eight out of 10 patients had trough concentration values below 10 mg/L at 48 hrs after a standard regimen treatment of patients with neutropenia due to underlying hematological disease. The initial inadequate concentrations during the first few days of therapy may affect the outcome of teicoplanin therapy with regard to the emergence of resistant microorganisms, and thus subtherapeutic concentrations should be avoided.
16 Especially in the critically ill patient setting, loading doses of teicoplanin (6 mg/kg every 12 hrs for at least three doses) should be considered mandatory in all patients and individually optimized dose regimens optimized. In addition, drug level monitoring is important during treatment.
17
Lothorary, et al.
12,
18 reported that patients with neutropenic fever have an increased distribution and elimination clearance without significant changes in the volume of distribution, as compared to healthy controls. Therefore, in this specific population, the trough concentration was associated with increased clearance and higher initial doses are required; administration of 6 mg/kg teicoplanin given every 12 hr for five doses ensured a mean trough serum concentration of 16.0 mg/L, with a trough concentration below 10 mg/L in 7% of patients, compared with 46% of patients on a regimen of teicoplanin given only four times during the first 48 h. According to Pea, et al.,
19 to ensure early therapeutically effective trough concentrations (more than 10 mg/L at 24 h) in patients with acute leukemia, a high loading regimen (800 mg+400 mg 12 hr apart on day 1,600 mg +400 mg 12 hr apart on day 2) followed by a high maintenance regimen (400 mg every 12 hr from day 3 on) showed successful attainment of the initial target.
It is known that glycopeptide antibiotics have a time-dependent killing pattern and moderate post-antibiotic effects. Thus, the ideal dosing regimen for a glycopeptide should be designed to maximize the amount of drug received. The AUC
24/MIC value could be used as the surrogate parameter associated with clinical efficacy. Unfortunately, PD data and AUC
24/MIC target value are not available for teicoplanin. Therefore, the values known for the vancomycin target value were adopted. Although past recommendations had indicated that at least an AUC
24/MIC >125 was necessary for vancomycin, recent studies recommend an AUC
24/MIC >345 or 400.
20-
23 Rybak, et al.
24 recommended that, for complicated infections caused by
S. aureus, trough vancomycin concentrations of 15-20 mg/L should be obtained and the range should achieve an AUC/MIC >400 for most patients if the MIC is <1 mg/L. However, an AUC/MIC of >400 is not attainable with conventional doses when the MIC is more than 2 mg/L in patients with normal renal function. In that situation, alternative therapies should be considered. Therefore, it is predicted that if the present study chose an AUC
24/MIC >400 as an attainable target, the TARs would show lower values than noted in the present results and larger teicoplanin doses would be needed for a favorable clinical outcome in this specific patient population with neutropenic fever. However, when the present study results were compared to clinical results from a study conducted at the same institution in which the clinical and microbiological response in febrile neutropenic patients were 53.3% and 62.5% for 200 mg/day maintenance dose regimen, the results using AUC
24/MIC >125 target was more consistent with clinical results than those from the AUC
24/MIC >345 target.
2 Based upon this correlation between AUC
24/MIC >125 target and clinical results, it might be inferred that to achieve 80% TAR, about 540 mg/day of teicoplanin would be required, and a corresponding adjustment of the standard dose regimen to 400 mg/day as a maintenance dose would be considered.
The MIC of teicoplanin for 90% of strains (MIC90) of S. aureus was reported to be below 1 mg/L3, while the MIC90 for S. aureus isolated from our HSCT center in this study was about 8-fold higher. Usual practice in Korea has been limited to low doses (400 mg single dose, then 200 mg once a day for empirical therapy; or 400 mg every 12 hrs for 3 doses, then 400 mg once a day for targeted therapy) due to the reimbursement system, which may contribute to the high MIC value for S. aureus.
Kuti, et al.
25 reported that a dose of 400 mg every 24 hr achieved a trough value >10 mg/L in 46.3% of the population studied. This would be similar to a 50% likelihood of a total AUC/MIC ratio >345 at an MIC of 1 mg/L. Similarly, the 800 mg dose achieved the target trough and AUC/MIC ratios in 83% and 94% of the population, respectively. In the present study, due to the high frequency of MIC >1 mg/L, the AUC
24/MIC >345 was not associated with MIC-nonspecific targets.
The results of the present study show that TARs at SS were only related to maintenance doses due to the PK properties of the PK/PD marker. With respect to the Ctrough >10 mg/L target, the standard recommended regimen, i.e., 400 mg q 12 hr iv for 3 doses then 400 mg once a day, showed that the current loading dose was insufficient to achieve SS-TAR during the early period of treatment in neutropenic patients. However, in the case of the AUC24/MIC, TAR achieved an earlier SS-TAR with the standard regimen compared to the MIC-nonspecific targets.
The logistic regression analysis for TAR of AUC
24/MIC ratio >345 with log (maintenance dose), suggested a higher dose was required than in previous reports for an adequate clinical success rate in the patient population. With respect to the emergence of resistance, the MIC breakpoint or dose regimen needs to be adjusted; however, there has been no definite consensus or guidelines established to date.
26
In conclusion, the current standard dose regimen (400 mg q 12 hr for 3 doses then, 400 mg once a day, iv) is predicted to be insufficient to treat S. aureus in Korean patients with neutropenic fever when the AUC24/MIC >345 was chosen as a PK/PD target to predict the clinical outcome. To assure at least an 80% TAR in this population, over 1,000 mg/day of teicoplanin as a maintenance dose is required and dose adjustment of teicoplanin would be considered. The limitations of the present study were that PK parameters from the literature were adopted instead of being based on the values of the patients in the study and the MIC values from single hospital in Korea were used. Therefore, additional well-designed prospective PK/PD outcome studies are needed to guide the optimal dosing regimens for specific patient populations.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



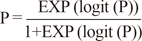




 XML Download
XML Download